On a piece of scratch paper, draw the Lewis structure for the SO32 ion. Then choose the correct Lewis structure for SO3² from the options listed below. (A) :O: :S: 0: :O: -2 (B) :O :S::O: :O: -2 (C) :O :S::O: :0: -2 (D) :O :S: 0: :O:
3.
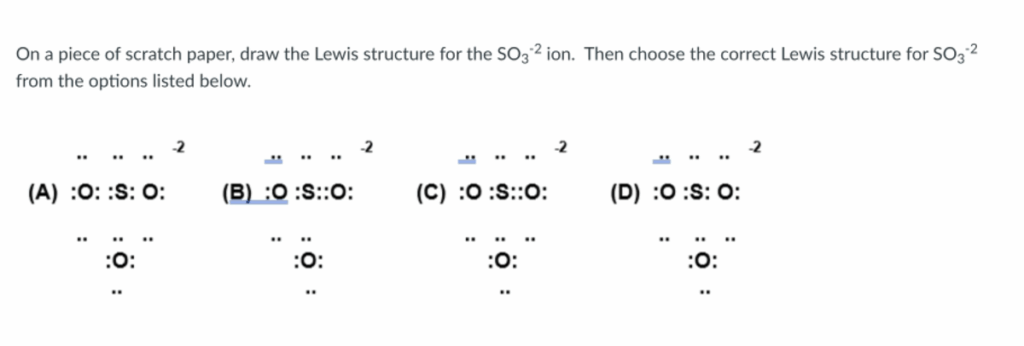
On a piece of scratch paper, draw the Lewis structure for the SO32 ion. Then choose the correct Lewis structure for SO3² from the options listed below. (A) :O: :S: 0: :0: -2 (B) :O :S::O: :O: -2 (C) :O :S::O: :0: -2 (D) :O :S: 0: :O:
The Correct Answer and Explanation is:
The correct Lewis structure for the sulfite ion SO32−\text{SO}_3^{2-} is option (B).
✅ Correct Answer: (B)
🔍 Explanation
To determine the correct Lewis structure of the sulfite ion SO32−\text{SO}_3^{2-}, we need to follow a systematic approach:
Step 1: Count Valence Electrons
- Sulfur (S) = 6 valence electrons
- Oxygen (O) = 6 × 3 = 18 valence electrons
- 2 extra electrons for the −2-2 charge
Total = 6 + 18 + 2 = 26 valence electrons
Step 2: Connect the Atoms
- Sulfur is the central atom because it’s less electronegative.
- Connect the three oxygens to the sulfur with single bonds initially.
Step 3: Complete Octets
- Place lone pairs on the oxygen atoms to complete their octets.
- Each single-bonded oxygen needs three lone pairs (6 electrons).
- This uses up 3×6=183 \times 6 = 18 electrons on the oxygens + 3×2=63 \times 2 = 6 electrons in bonds = 24 electrons.
We have used 24 of 26 electrons so far. The remaining 2 electrons go on the sulfur atom as a lone pair.
Step 4: Formal Charges
- We want to minimize formal charges and distribute the negative charge over the most electronegative atoms (oxygen).
In structure (B):
- One oxygen is double bonded to sulfur (formal charge 0).
- Two oxygens are single bonded and carry a -1 formal charge each.
- Sulfur has a formal charge of 0.
This gives a total charge of -2, which matches the ion’s charge.
Step 5: Resonance
Although the drawing shows one double bond, in reality, SO32−\text{SO}_3^{2-} has resonance structures, meaning the double bond can be with any of the three oxygens, averaging bond character.
✔ Conclusion:
Option (B) is correct because:
- It uses the correct number of valence electrons.
- It obeys the octet rule.
- It minimizes formal charges.
- It reflects proper resonance possibility
