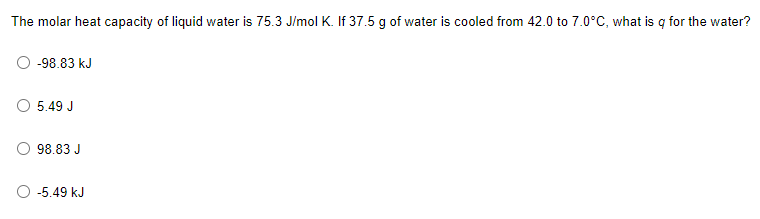
The molar heat capacity of liquid water is 75.3 J/mol K. If 37.5 g of water is cooled from 42.0 to 7.0°C, what is q for the water?
- -98.83 kJ
- 5.49 J
- 98.83 J
- -5.49 kJ
The Correct Answer and Explanation is:
To solve this problem, we use the formula for heat transfer: q=n⋅Cm⋅ΔTq = n \cdot C_m \cdot \Delta T
Where:
- qq is the heat (in joules),
- nn is the number of moles of water,
- CmC_m is the molar heat capacity of water (given as 75.3 J/mol·K),
- ΔT\Delta T is the change in temperature (final – initial).
Step-by-step:
- Calculate moles of water: Mass of water=37.5 g,Molar mass of water=18.015 g/mol\text{Mass of water} = 37.5\ \text{g},\quad \text{Molar mass of water} = 18.015\ \text{g/mol} n=37.5 g18.015 g/mol≈2.082 moln = \frac{37.5\ \text{g}}{18.015\ \text{g/mol}} \approx 2.082\ \text{mol}
- Calculate temperature change: ΔT=Tfinal−Tinitial=7.0∘C−42.0∘C=−35.0∘C=−35.0 K\Delta T = T_{\text{final}} – T_{\text{initial}} = 7.0^\circ C – 42.0^\circ C = -35.0^\circ C = -35.0\ \text{K}
- Calculate heat qq: q=2.082 mol⋅75.3 J/mol\cdotpK⋅(−35.0 K)=−5491.6 Jq = 2.082\ \text{mol} \cdot 75.3\ \text{J/mol·K} \cdot (-35.0\ \text{K}) = -5491.6\ \text{J}
- Convert to kilojoules: q=−5491.6 J=−5.49 kJq = -5491.6\ \text{J} = -5.49\ \text{kJ}
Final Answer:
✔️ -5.49 kJ
Explanation:
The water is cooling down, which means it is losing heat, so qq is negative. We used the molar heat capacity because the question provides it in units of J/mol·K. By converting the mass of water to moles and multiplying by the molar heat capacity and temperature change, we determine the total energy lost by the water during the cooling process.
Among the choices:
- “-5.49 kJ” is the correct value and sign,
- The other options are either in the wrong units (J instead of kJ), have the wrong magnitude, or have the wrong sign.
This calculation is crucial in thermodynamics and chemistry for understanding how substances absorb or release energy with temperature changes.
