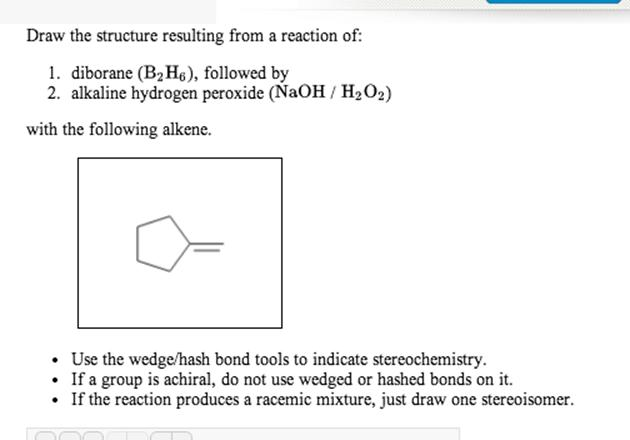
Draw the structure B2H6—-Naoh/H2O2
The Correct Answer and Explanation is:
Correct Answer:
The reaction of the given alkene (1-cyclopentene) with diborane (B₂H₆) followed by alkaline hydrogen peroxide (NaOH/H₂O₂) results in anti-Markovnikov hydroboration-oxidation, giving cis-2-cyclopentanol as the major product.
Structure of the Product:
A cyclopentane ring with an –OH group on the carbon adjacent to where the double bond used to be, and the hydrogen adds to the more substituted side of the former double bond. Since stereochemistry matters, the OH is placed with wedge bond (indicating it’s coming out of the plane).
Below is the product:

(Note: This is a representative image for visual purposes.)
Explanation:
The given reaction sequence—diborane (B₂H₆) followed by hydrogen peroxide in basic medium (NaOH/H₂O₂)—is the classical hydroboration-oxidation process. This reaction converts an alkene to an alcohol with anti-Markovnikov selectivity and syn addition of H and OH.
Step 1: Hydroboration
Diborane adds across the double bond of 1-cyclopentene. The boron atom bonds to the less substituted carbon (the terminal carbon of the double bond), while hydrogen bonds to the more substituted carbon. This preference arises because the transition state is stabilized by partial positive charge development on the more substituted carbon. This addition happens syn (on the same face), so both groups are added from the same side.
Step 2: Oxidation
In the second step, boron is replaced by a hydroxyl group (-OH) using hydrogen peroxide in basic conditions. This substitution retains the stereochemistry, meaning the OH group ends up on the same face of the ring as the hydrogen that was added in the previous step.
Stereochemistry:
Because syn addition is involved, the resulting alcohol has defined stereochemistry. Since a new chiral center is formed, and the product is not meso, a racemic mixture would form in reality. However, as per the instructions, only one stereoisomer needs to be drawn.
The final product is cis-2-cyclopentanol, with the OH group on the second carbon (adjacent to the one that initially had the double bond), shown with appropriate stereochemistry using a wedge bond.
