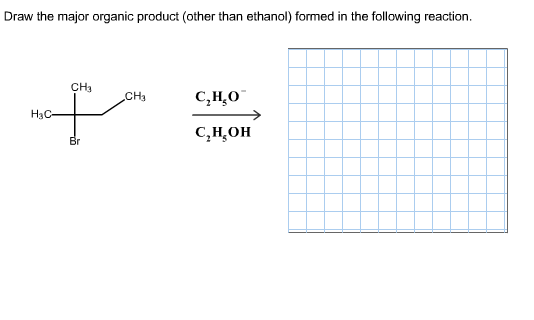
Draw the major organic product formed in the following reaction
The Correct Answer and Explanation is:
Correct Answer:
The major organic product of the given reaction is:
2-methyl-2-butene

Explanation (Approximately 300 words):
The starting compound is 2-bromo-2-methylbutane, and it is treated with ethoxide ion (C₂H₅O⁻) in ethanol (C₂H₅OH). This setup strongly suggests an E2 elimination reaction.
In E2 reactions:
- A strong base (like ethoxide) abstracts a β-hydrogen (a hydrogen on the carbon adjacent to the one bearing the leaving group).
- Simultaneously, the leaving group (bromine in this case) leaves, forming a double bond.
Step-by-step Breakdown:
- Structure of the substrate:
The molecule is 2-bromo-2-methylbutane. The central carbon (C2) is bonded to:- A bromine atom (leaving group),
- A methyl group,
- An ethyl group,
- Another methyl group.
- Identification of β-hydrogens:
The β-hydrogens are on the adjacent carbon atoms to C2:- The carbon on the ethyl side (C3) has hydrogens available for elimination.
- The methyl groups do not have β-hydrogens that would lead to stable alkenes.
- Major product prediction using Zaitsev’s Rule:
Zaitsev’s Rule states that in elimination reactions, the more substituted (more stable) alkene will be the major product.- Elimination of a β-hydrogen from C3 results in the formation of 2-methyl-2-butene, a more substituted alkene (disubstituted).
- Elimination from the other β-position would give 2-methyl-1-butene (less substituted), which is the minor product.
- Conclusion:
The major organic product formed is 2-methyl-2-butene, resulting from the E2 elimination of H from C3 and Br from C2, forming a double bond between C2 and C3.
This reaction is a textbook example of a base-promoted E2 elimination leading to an alkene as the major organic product.
