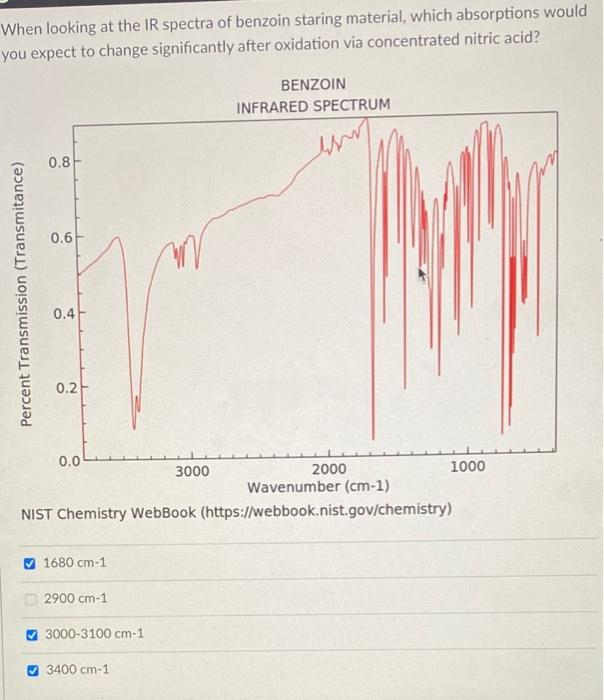
When looking at the IR spectra of benzoin staring material, which absorptions would you expect to change significantly after oxidation via concentrated nitric acid? Percent Transmission (Transmitance)
- 0.8
- 0.6
- 0.4
- 0.2
- 0.0
1680 cm-1 2900 cm-1 3000-3100 cm-1 3000 3400 cm-1 BENZOIN Wavenumber (cm-1) NIST Chemistry WebBook (https://webbook.nist.gov/chemistry) INFRARED SPECTRUM
- 2000
- 1000
The Correct Answer and Explanation is:
Correct Answer:
✅ 1680 cm⁻¹
✅ 3400 cm⁻¹
Explanation
The infrared (IR) spectrum of benzoin, a molecule containing both a hydroxyl (-OH) group and a carbonyl (C=O) group, shows characteristic absorptions that correspond to these functional groups.
In the IR spectrum:
- The broad peak around 3400 cm⁻¹ corresponds to the O–H stretching vibration from the hydroxyl group in benzoin.
- The absorption around 1680 cm⁻¹ is due to the C=O stretching vibration of the ketone group in benzoin.
When benzoin is oxidized using concentrated nitric acid, it is typically converted into benzil, which contains two ketone (C=O) groups but no hydroxyl group.
As a result, the following changes are expected in the IR spectrum:
- 3400 cm⁻¹ (O–H stretch):
- This peak will disappear or significantly decrease because the hydroxyl group is oxidized to a carbonyl group.
- Therefore, this absorption will change significantly after oxidation.
- 1680 cm⁻¹ (C=O stretch):
- The intensity of this peak will increase, and the position may shift slightly depending on conjugation and environment, since there will be two C=O groups instead of one.
- This region will change significantly due to increased carbonyl content.
On the other hand:
- 2900 cm⁻¹: This corresponds to C–H stretching, which is not significantly affected by oxidation.
- 3000–3100 cm⁻¹: This region typically corresponds to aromatic C–H stretches and may show little to no significant change during oxidation since the aromatic system remains intact.
Conclusion:
The major spectral changes occur in the 1680 cm⁻¹ and 3400 cm⁻¹ regions due to the oxidation of the hydroxyl group to a second carbonyl. Thus, those are the correct regions to expect significant changes after oxidation.
