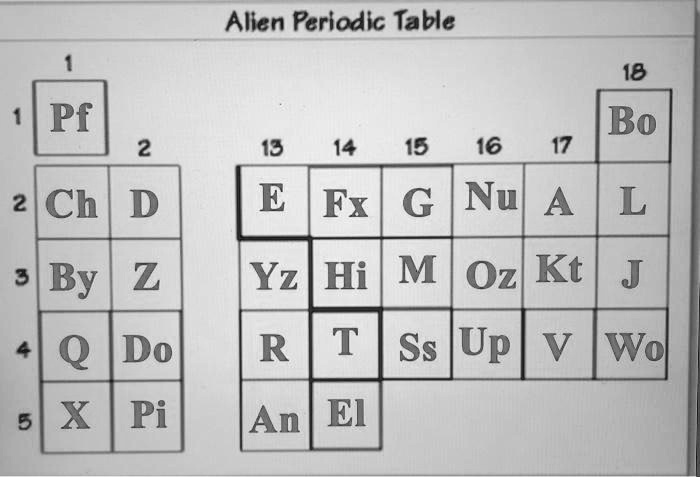
Alien Periodic Table 1 18 1 Pf Bo 2 13 14 15 16 17 2 Ch D E Fx G Nu A L 3 By Z
The Correct Answer and Explanation is :
The image shows a fictional “Alien Periodic Table” with element symbols that resemble—but do not exactly match—the standard periodic table of elements. The goal of such puzzles is typically to match the alien elements to their real-world counterparts based on their positions and periodic trends.
Correct Answer:
This is a conceptual matching puzzle. The element in the top left, “Pf”, is in Group 1, Period 1, which corresponds to Hydrogen (H). The rest can be deduced similarly by matching positions.
Explanation
This “Alien Periodic Table” mimics the layout of the real periodic table, using fictional symbols like “Pf”, “Ch”, “By”, etc. Despite the alien letters, the structure is key. The periodic table is organized by groups (columns) and periods (rows). Elements in the same group share similar chemical properties, and elements across a period show gradual changes in properties.
To solve such puzzles, we rely on periodic trends and the positions of elements, not their names.
- Group 1, Period 1 contains Hydrogen (H) – on this alien table, that’s “Pf”.
- Group 18, Period 1 contains Helium (He) – that’s “Bo”.
- Group 1, Period 2 would be Lithium (Li) – in this table, “Ch”.
- Group 2, Period 2 would be Beryllium (Be) – “D”.
- Group 13-18, Period 2 would be elements like Boron (B) to Neon (Ne) – mapped as E, Fx, G, Nu, A, L.
- Continue with Period 3: By (Na), Z (Mg), etc.
- The transition metals appear in the central block (Groups 3–12 in standard tables), though this simplified layout omits them.
Understanding the periodicity of element properties like atomic number, reactivity, and electron configuration helps decode which alien symbols match real elements. The vertical columns (groups) indicate similar valence electron configurations, so elements like “A”, “Kt”, and “V” in Group 17 likely correspond to halogens (F, Cl, Br).
This exercise teaches periodic trends, electron configuration, and group/period relationships—essential concepts in chemistry.
