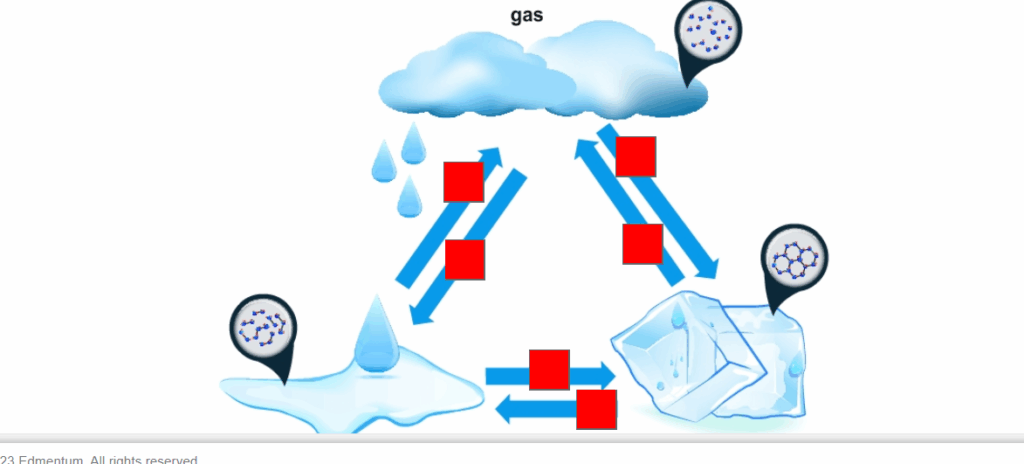
Select the correct locations on the image. Identify the arrows that represent the process of cooling.
The Correct Answer and Explanation is:
In the image provided, the arrows that represent the process of cooling are those that move from a higher energy state to a lower energy state:
- From gas to liquid – This is condensation.
- From liquid to solid – This is freezing.
Correctly Identified Arrows:
- The downward arrow from the cloud (gas) to the water droplets (liquid).
- The rightward arrow from the puddle of liquid to the ice cube (solid).
Explanation
Cooling is a physical process in which a substance loses thermal energy, causing its particles to slow down and move closer together. This typically results in a change of state from a higher energy form (like gas) to a lower energy form (like liquid or solid).
In the provided diagram, we observe the water cycle and the different states of matter: gas (clouds), liquid (water droplets or puddle), and solid (ice). When a gas turns into a liquid, it undergoes condensation. This happens when water vapor in the air cools down and loses energy. The particles slow down and come closer together, forming liquid water. This is represented by the downward arrow from the cloud to the falling droplets.
Similarly, when liquid water becomes a solid (like freezing a puddle into ice), it undergoes freezing. In this process, the liquid loses even more energy, and its molecules arrange themselves into a fixed, orderly structure characteristic of a solid. This is shown by the rightward arrow from the puddle to the ice cube.
Both condensation and freezing are exothermic processes, meaning they release heat to the surroundings. As such, these transitions are associated with cooling, and they are vital in natural processes like cloud formation and the freezing of water in cold environments.
Identifying these arrows correctly is important in understanding phase changes and energy transfer in matter. The opposite transitions—melting (solid to liquid) and evaporation (liquid to gas)—involve heating, not cooling.
