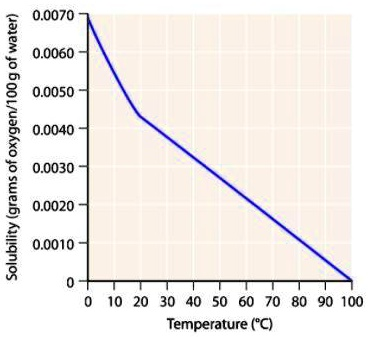
Solubility (grams of oxygen/100 g of water) 0.0070 0.0060 0.0050 0.0040 0.0030 0.0020 0.0010 0 0 10 20 30 40 50 60 70 80 90 100 Temperature (°C)
The Correct Answer and Explanation is:
Correct Answer:
As the temperature increases, the solubility of oxygen in water decreases.
Explanation
The graph provided illustrates the relationship between temperature (in °C) and the solubility of oxygen (in grams per 100 g of water). It clearly shows a negative correlation between temperature and oxygen solubility—meaning that as temperature increases, the solubility of oxygen decreases.
At 0°C, the solubility of oxygen is at its highest, approximately 0.0070 g/100 g of water. As the temperature increases to 20°C, solubility decreases to about 0.0045 g/100 g of water. By the time the temperature reaches 100°C, the solubility has dropped dramatically to nearly 0.000 g/100 g of water. This trend indicates that warmer water holds less dissolved oxygen.
This behavior can be explained by molecular motion and gas-liquid interactions. At lower temperatures, water molecules move more slowly, allowing more oxygen molecules to remain dissolved in the liquid. As the temperature increases, molecular movement becomes more vigorous, and oxygen molecules are more likely to escape from the liquid phase into the air, thus decreasing solubility.
This phenomenon has important environmental and biological implications. For instance, aquatic life depends on dissolved oxygen for survival. In warmer water bodies (especially during summer or due to climate change), oxygen levels can drop, leading to hypoxic conditions—areas with too little oxygen to support most marine life.
In addition, this principle is critical in industrial and laboratory settings. Processes that require high dissolved oxygen levels, such as aquaculture, wastewater treatment, and bioreactors, must consider temperature as a key variable to optimize oxygen availability.
In summary, the graph shows that the solubility of oxygen in water decreases as temperature increases, due to the physical properties of gases and their interactions with liquid water.
