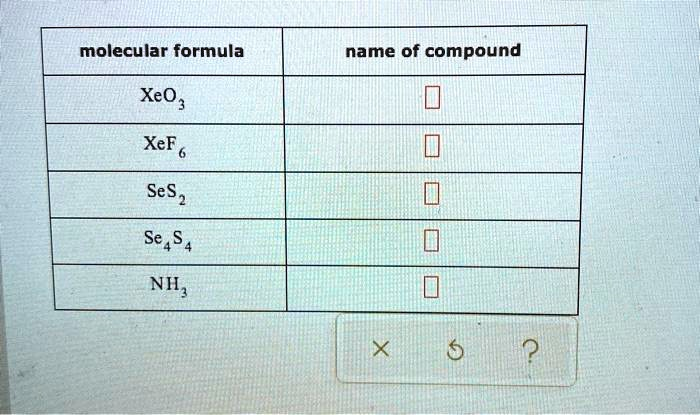
Molecular formula Name of compound XeO2 XeF2 SeS2 Se4S4 Ni
The Correct Answer and Explanation is:
| Molecular Formula | Name of Compound |
|---|---|
| XeO₃ | Xenon trioxide |
| XeF₆ | Xenon hexafluoride |
| SeS₂ | Selenium disulfide |
| Se₄S₄ | Tetraselenium tetrasulfide |
| NH₃ | Ammonia |
Explanation
The naming of chemical compounds depends on whether they are ionic or molecular. The compounds in this list are molecular compounds, formed between nonmetals, and are named using prefixes to indicate the number of atoms.
- XeO₃ – Xenon trioxide:
Xenon is a noble gas that can form compounds with electronegative elements like oxygen. In XeO₃, there are three oxygen atoms bonded to xenon. The prefix “tri-” means three, and “oxide” is used for oxygen in binary compounds. Therefore, the correct name is xenon trioxide. - XeF₆ – Xenon hexafluoride:
Similar to XeO₃, this compound involves xenon and six fluorine atoms. “Hexa-” is the prefix for six, and “fluoride” indicates fluorine. Thus, it is named xenon hexafluoride. - SeS₂ – Selenium disulfide:
This is a compound of selenium and sulfur. The “di-” prefix before “sulfide” indicates two sulfur atoms. Since selenium is less electronegative than sulfur, it is written first. The correct name is selenium disulfide. - Se₄S₄ – Tetraselenium tetrasulfide:
This compound has four selenium and four sulfur atoms. Prefixes “tetra-” are used for both elements. Therefore, it is named tetraselenium tetrasulfide. - NH₃ – Ammonia:
This is a special case. While its molecular formula is nitrogen trihydride, the compound is commonly known by its traditional or common name, ammonia. Such traditional names are often used for well-known compounds.
In conclusion, correct naming requires attention to chemical conventions, especially prefixes for molecular compounds and recognition of common/traditional names.
