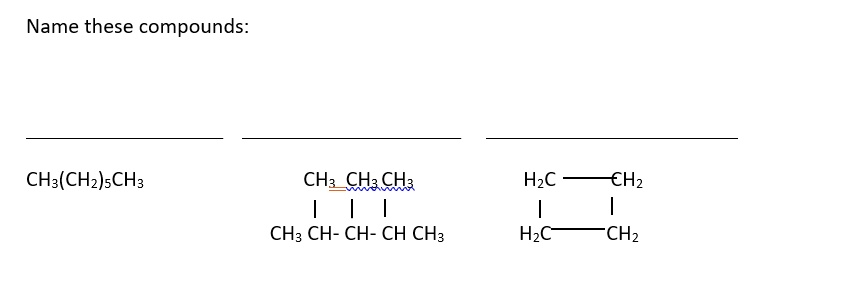
Name these compounds: CH
(CH
)
CH
CH
CH
CH
| | | CH
CH
CH
CH CH
H
C
CH
| | H
C
CH
Name these compounds: CH
(CH
)
CH
CH
CH
CH
| | | CH
CH
CH
CH CH
H
C
CH
| | H
C
CH
The Correct Answer and Explanation is:
Let’s name the three organic compounds from the image:
1. CH₃(CH₂)₅CH₃
Name: n-heptane
Explanation:
This is a straight-chain alkane with 7 carbon atoms. The general formula for an alkane is CₙH₂ₙ₊₂. Counting the carbon atoms:
- 1 carbon in CH₃ (methyl)
- 5 carbons in (CH₂)₅
- 1 carbon in the terminal CH₃
This adds up to 7 carbons → heptane.
The “n-” prefix (normal) denotes a straight-chain alkane with no branching.
2. Branched hydrocarbon:
CH3
|
CH3—CH—CH—CH—CH3
| |
CH3 CH3
Name: 2,3,4-trimethylpentane
Explanation:
- Longest continuous carbon chain: 5 carbon atoms → pentane
- Methyl groups (–CH₃) are attached at positions 2, 3, and 4
- Number the chain from the end closest to a substituent to get the lowest set of locants: 2,3,4
- Three methyl groups → trimethyl
Final name: 2,3,4-trimethylpentane
3. Four-carbon ring:
CH2—CH2
| |
CH2 CH2
Name: cyclobutane
Explanation:
This structure is a closed ring with 4 carbon atoms, all single bonds → it’s a cycloalkane.
- 4 carbon atoms → butane
- Ring structure → prefix cyclo-
Final name: cyclobutane
Summary Table:
| Compound Structure | Name |
|---|---|
| CH₃(CH₂)₅CH₃ | n-heptane |
| Branched chain with 3 methyl groups | 2,3,4-trimethylpentane |
| Four-carbon ring | cyclobutane |
Additional Explanation
The naming of organic compounds follows IUPAC nomenclature rules. Alkanes are saturated hydrocarbons containing only single bonds. Naming them involves identifying the longest carbon chain and then naming and numbering any branches or substituents.
- For n-heptane, the molecule is linear and unbranched. The base name “heptane” comes from the 7 carbon atoms, and “n-” means it’s the normal (straight) form. No prefixes are needed beyond “n-” since there are no branches.
- The second compound is a branched alkane. The longest chain of carbon atoms is selected (pentane), and the branches (methyl groups) are identified and numbered to give the lowest possible numbers. This ensures the name is unique and systematic. Since there are methyl groups on carbons 2, 3, and 4, the name becomes 2,3,4-trimethylpentane.
- The third compound is a cyclic hydrocarbon, specifically a cycloalkane. The ring contains four carbon atoms, all bonded with single bonds, making it cyclobutane. The “cyclo-” prefix indicates a ring, while “butane” indicates four carbons.
Understanding these naming rules helps chemists clearly describe molecular structures in a consistent, universally understood way.
