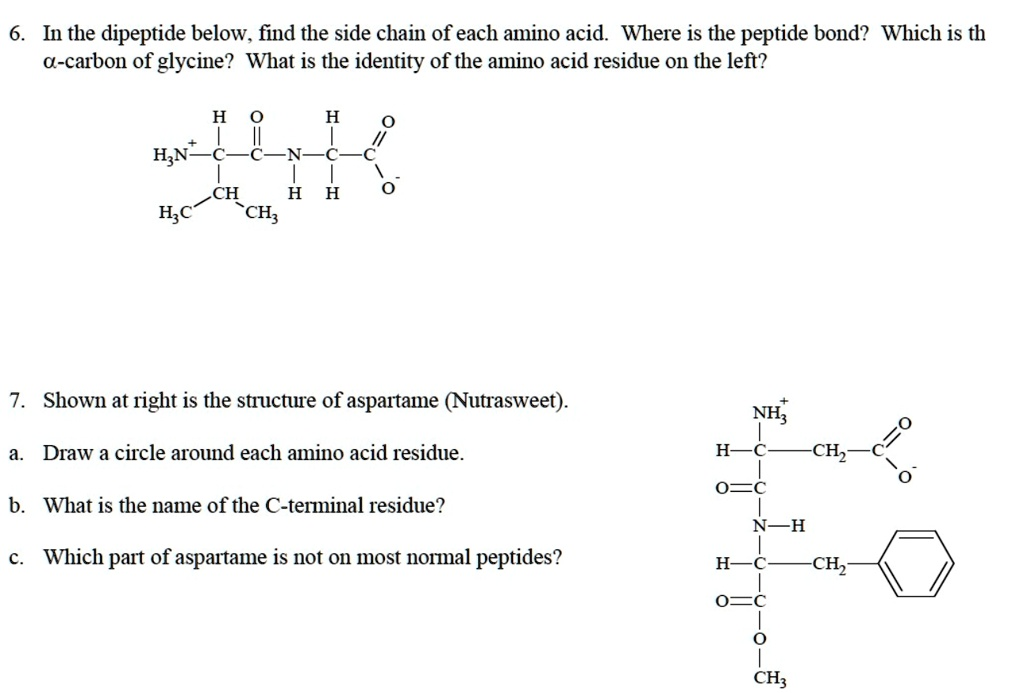
In the dipeptide below, find the side chain of each amino acid. Where is the peptide bond? Which is the γ-carbon of glycine? What is the identity of the amino acid residue on the left? H3N+-C-C-C-C-C-COO- | | | | | | H H H H H H Shown at right is the structure of aspartame (Nutrasweet). NH2 Draw a circle around each amino acid residue. H -CH2 O=C What is the name of the C-terminal residue? NH2 Which part of aspartame is not in most normal peptides? H O CH3 -CH2
The Correct Answer and Explanation is:
6. In the dipeptide below:
a. Find the side chain of each amino acid:
The two amino acids in the dipeptide are:
- Left amino acid (with side chain -CH(CH₃)₂): This is valine. Its side chain is isopropyl (–CH(CH₃)₂).
- Right amino acid (with side chain -H): This is glycine, which has a hydrogen atom as its side chain.
b. Where is the peptide bond?
The peptide bond is between the carbon of the first amino acid’s carboxyl group (C=O) and the nitrogen of the second amino acid’s amino group (NH). In the diagram, it’s the bond between the C=O of valine and the NH of glycine.
c. Which is the α-carbon of glycine?
The α-carbon of glycine is the carbon attached to both the amino group (–NH₂) and the carboxyl group (–COOH). In glycine, this carbon also has two hydrogen atoms as its side chain.
d. What is the identity of the amino acid residue on the left?
The left amino acid residue is valine, identifiable by its isopropyl side chain.
7. Structure of Aspartame (Nutrasweet):
a. Draw a circle around each amino acid residue:
Aspartame contains two amino acid residues:
- Aspartic acid – on the left (NH₃⁺, CH₂, COOH)
- Phenylalanine – on the right (CH₂ connected to a phenyl group)
b. What is the name of the C-terminal residue?
The C-terminal residue is phenylalanine. It ends in a methyl ester rather than a free carboxyl group.
c. Which part of aspartame is not in most normal peptides?
The part that is not found in most peptides is the methyl ester group (–COOCH₃) on the C-terminus. Most peptides end with a free carboxyl group (–COOH), not an esterified one.
Explanation
In biochemistry, understanding peptide structure is critical. Peptides are chains of amino acids linked by peptide bonds, which form between the carboxyl group of one amino acid and the amino group of the next. The side chains (R groups) determine the identity of each amino acid. In the dipeptide shown, valine is on the left with a branched isopropyl side chain, while glycine is on the right with a single hydrogen side chain. The peptide bond is the central link between the two amino acids and is critical for protein structure. Each amino acid also has a unique α-carbon, the central carbon to which the amino, carboxyl, hydrogen, and side chain are attached. For glycine, this α-carbon is distinctive as it binds only hydrogens.
In aspartame, a commercial sweetener, the peptide-like structure contains two amino acids: aspartic acid and phenylalanine. These are connected similarly to a dipeptide, but the C-terminus of phenylalanine is modified into a methyl ester (–COOCH₃) to enhance sweetness and stability. This esterification distinguishes aspartame from typical peptides, which usually end in a free carboxyl group. Understanding these structural details not only helps in identifying amino acids and their residues but also in recognizing functional modifications in biologically relevant molecules like aspartame.
