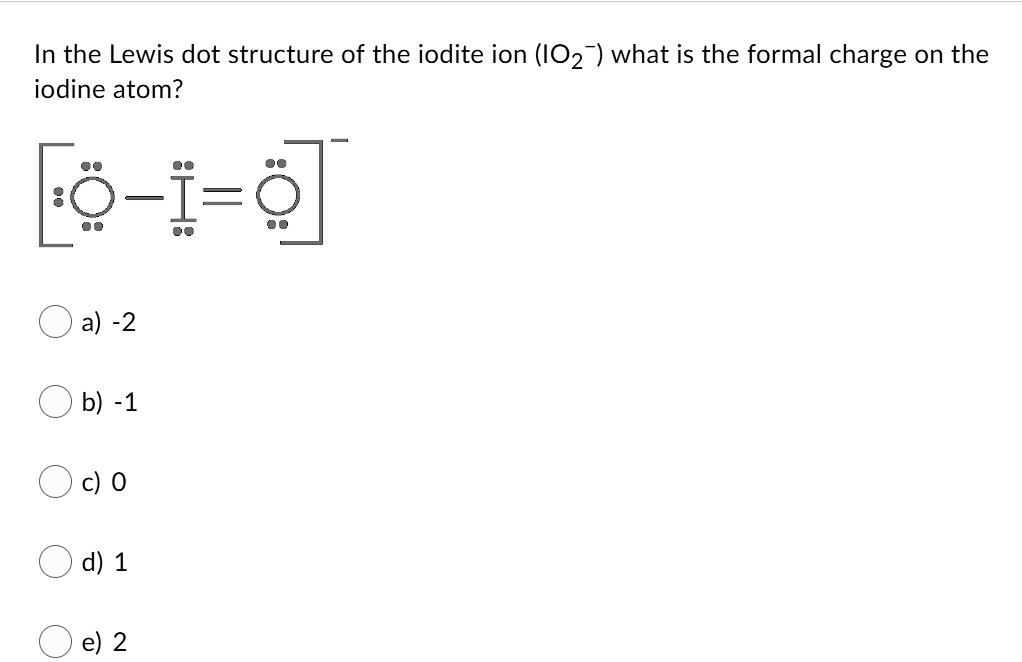
In the Lewis dot structure of the iodite ion (IO2-), what is the formal charge on the iodine atom? a) -2 b) -1 c) 0 d) 1 e) 2
The Correct Answer and Explanation is:
To find the formal charge on the iodine atom in the iodite ion (IO₂⁻), we use the formal charge formula:Formal charge=Valence electrons−Non-bonding electrons−12×Bonding electrons\text{Formal charge} = \text{Valence electrons} – \text{Non-bonding electrons} – \frac{1}{2} \times \text{Bonding electrons}Formal charge=Valence electrons−Non-bonding electrons−21×Bonding electrons
Step 1: Count valence electrons
Iodine (I) is in Group 17, so it has 7 valence electrons.
Step 2: Examine the Lewis structure
In the provided Lewis structure:
- Iodine is bonded to two oxygen atoms.
- One oxygen is connected with a single bond, and the other with a double bond.
- Iodine has two lone electrons (1 lone pair).
- Single bond = 2 electrons, double bond = 4 electrons → total bonding electrons around I = 6 electrons.
Step 3: Apply the formula
- Valence electrons = 7
- Non-bonding electrons (on iodine) = 2
- Bonding electrons = 6 → shared, so iodine gets 6/2 = 3
Formal charge=7−2−3=∗∗+2∗∗\text{Formal charge} = 7 – 2 – 3 = **+2**Formal charge=7−2−3=∗∗+2∗∗
Final Answer:
e) 2
Explanation
The formal charge is a bookkeeping tool in chemistry used to determine the distribution of electrons in a molecule or ion. It helps identify the most stable Lewis structure and determine which atoms carry partial charges. The formula used is:Formal charge=Valence electrons−Non-bonding electrons−12×Bonding electrons\text{Formal charge} = \text{Valence electrons} – \text{Non-bonding electrons} – \frac{1}{2} \times \text{Bonding electrons}Formal charge=Valence electrons−Non-bonding electrons−21×Bonding electrons
In the iodite ion (IO₂⁻), iodine is the central atom bonded to two oxygen atoms. Based on the Lewis structure in the image, one oxygen forms a single bond with iodine, while the other forms a double bond. The iodine atom also retains one lone pair (2 electrons). This structure gives iodine a total of 2 non-bonding electrons and 6 bonding electrons (from the 1 single bond and 1 double bond). Since bonding electrons are shared, iodine only “owns” half of them—3 electrons.
Subtracting the non-bonding electrons (2) and half of the bonding electrons (3) from iodine’s 7 valence electrons gives:7−2−3=+27 – 2 – 3 = +27−2−3=+2
This means iodine in IO₂⁻ carries a formal charge of +2. Note that the total structure carries a net charge of -1, which is due to the distribution of formal charges on the entire ion (including the negatively charged oxygen atoms). The structure is valid and obeys the octet rule for the atoms involved.
Thus, the correct answer is (e) 2.