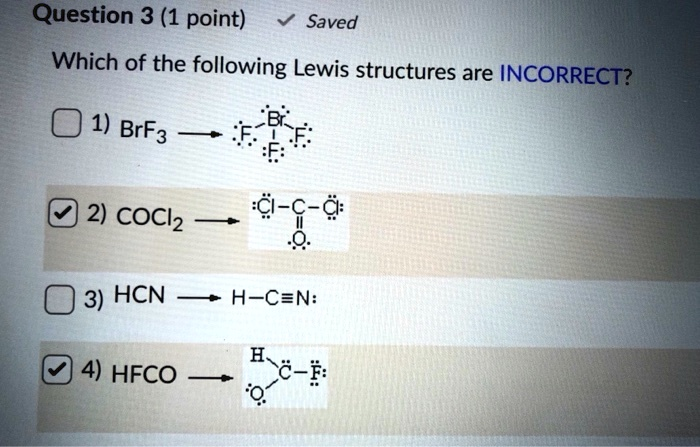
Saved Which of the following Lewis structures are INCORRECT? 1) BrF3 Br 2) COCl2 C-C-C: 3) HCN H-C=N: 4) HFCO E-C-# 1
The Correct Answer and Explanation is:
The question asks which Lewis structures are incorrect, and the correct answer is:
✅ 2) COCl₂ and 4) HFCO — both are incorrect.
Explanation
1) BrF₃ (Bromine trifluoride) – Correct
The Lewis structure for BrF₃ is accurate. Bromine (Br) is the central atom with 3 bonded fluorine atoms and 2 lone pairs. This leads to 5 electron regions, which gives it a T-shaped molecular geometry, matching the Lewis structure shown.
2) COCl₂ (Phosgene) – Incorrect
The shown structure has two carbon atoms, which is incorrect. COCl₂ should have one central carbon atom double-bonded to one oxygen atom and single-bonded to two chlorine atoms. The correct Lewis structure is:
mathematicaCopyEdit O
||
Cl—C—Cl
- Carbon has 4 bonds (full octet).
- Oxygen has a double bond and two lone pairs.
- Chlorines have three lone pairs each.
The structure in the image incorrectly shows C–C–C bonding, which does not represent phosgene. Therefore, it’s incorrect.
3) HCN (Hydrogen cyanide) – Correct
The given Lewis structure for HCN is:
mathematicaCopyEditH—C≡N:
- Hydrogen forms 1 bond.
- Carbon forms 4 bonds (1 with H, 3 with N).
- Nitrogen has a triple bond and one lone pair.
This is correct.
4) HFCO (Formyl fluoride) – Incorrect
The image incorrectly shows a single bond between C and O and possibly incorrect bonding arrangements. The correct structure is:
scssCopyEditH—C(=O)—F
- Carbon forms 4 bonds: 1 to H, 1 to F, and 2 to O (double bond).
- Oxygen has 2 lone pairs.
- Fluorine has 3 lone pairs.
The image structure appears to violate the octet rule and bonding conventions, so it’s incorrect.
Final Answer:
✅ 2 and 4 are incorrect Lewis structures.
