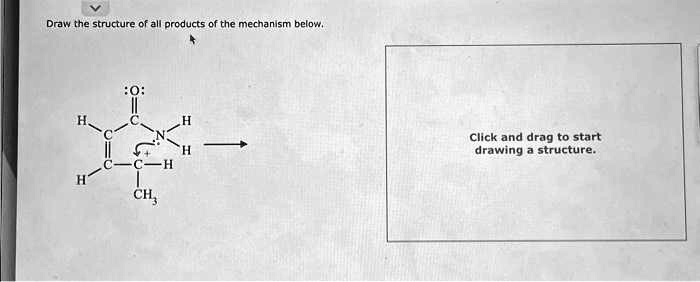
Draw the structure of all products of the mechanism below. :O: H C H C N H C C-H H CH
Click and drag to start drawing a structure.
The Correct Answer and Explanation is:
The image depicts a molecular structure undergoing a reaction, with a lone pair on an oxygen atom and a curved arrow indicating electron movement from a carbon-carbon double bond to a neighboring nitrogen. Let’s analyze and determine the products.
Step-by-Step Mechanism Analysis
The reactant is a β-keto imine, with a structure as follows:
mathematicaCopyEditH O
| ||
C = C - C - N - H
| |
CH3 H
A curved arrow points from the π electrons of the C=C double bond toward the nitrogen, suggesting a tautomerization or rearrangement, possibly resulting in a Michael-type addition or enamine-imine tautomerization.
- Electron Flow:
- The π electrons from the C=C bond move toward the nitrogen, which already has a lone pair.
- This causes the C-N bond to become a double bond (C=N).
- The nitrogen donates a hydrogen to the adjacent carbon (a proton shift), resulting in:
- Formation of a C=N double bond.
- The former double bond carbon becomes single-bonded with an additional H (protonated).
- New Bonds Formed:
- The enamine tautomer shifts to an imine.
- The ketone remains unchanged.
Final Product Structure
The structure of the product is:
mathematicaCopyEdit O
||
H3C–C–CH2–C=N–H
Here’s what happened:
- The double bond between the second and third carbon has shifted.
- The nitrogen now forms a double bond with the fourth carbon (C=N).
- The hydrogen has shifted to the third carbon, converting it to a methylene (-CH2-).
Explanation
The reaction shown involves a tautomerization mechanism, specifically an enamine to imine shift. The starting compound contains a conjugated system with a carbon-carbon double bond adjacent to a nitrogen (with a hydrogen) and a ketone group (C=O). These functional groups allow for electron delocalization and proton transfers, stabilizing new resonance structures or tautomers.
The curved arrow indicates the movement of electrons from the C=C double bond to form a new C=N bond. When the double bond electrons shift toward the nitrogen, it creates a negative charge on nitrogen temporarily. To stabilize this intermediate, the nitrogen donates a proton (H⁺) to the adjacent carbon that lost its double bond character. This proton transfer forms a neutral species and results in the final product—a stable imine structure with a C=N bond and a shifted methylene (-CH2-) group.
This type of transformation is common in organic chemistry, especially in the chemistry of α,β-unsaturated carbonyl compounds and enamines. Such rearrangements are essential in many biological systems and synthetic pathways, as they can alter molecular reactivity and enable further transformations like nucleophilic addition.
Overall, the mechanism involves:
- Electron delocalization through π bonds.
- Formation of an imine (C=N).
- Proton transfer to maintain charge balance.
The product is stable and no longer contains the original C=C double bond—it has been replaced by a C=N double bond, completing the tautomerization.
