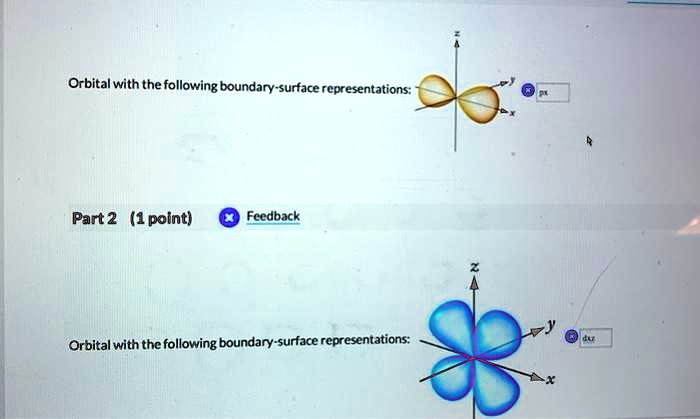
Orbital with the following boundary-surface representations: Part 2 (1 point) Feedback Orbital with the following boundary-surface representations:
The Correct Answer and Explanation is:
Correct Answers:
- Top Orbital (Orange, dumbbell shape along x-axis):
Correct answer:pxorbital - Bottom Orbital (Blue, four-lobed shape in the xy-plane):
Correct answer:d(xy)orbital
Explanation
Atomic orbitals are regions around the nucleus where the probability of finding an electron is high. These orbitals are visualized as boundary-surface diagrams that represent where electrons are likely to be located 90% of the time.
The first image shows an orbital with a dumbbell shape oriented along the x-axis. This is characteristic of a 2p orbital, specifically the px orbital. The “p” orbitals (px, py, pz) all have similar dumbbell shapes, but they are oriented along different axes (x, y, and z, respectively). Each p orbital has two lobes with a node (a region of zero probability) at the nucleus. In this case, the orientation along the x-axis clearly indicates that it is a px orbital.
The second image depicts a four-lobed orbital lying in the xy-plane, characteristic of a d orbital. Among the five d orbitals (dxy, dxz, dyz, dx²−y², dz²), the dxy orbital has its lobes situated between the x and y axes — not aligned with them but between them. The cloverleaf appearance in the xy-plane confirms this as a dxy orbital. Unlike p orbitals, d orbitals have more complex shapes and contain two nodal planes where the probability of finding an electron is zero.
Understanding orbital shapes is crucial for interpreting chemical bonding, electron configurations, and molecular geometry. These visualizations help predict how atoms interact in chemical reactions and how molecules form specific shapes based on orbital hybridization.
