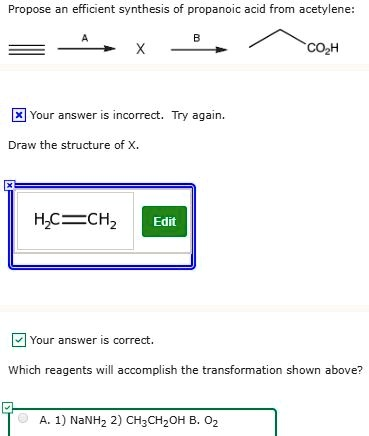
Propose an efficient synthesis of propanoic acid from acetylene: COOH Your answer incorrect. Try again. Draw the structure of X, HC≡CH Edit Your answer correct. Which reagents will accomplish the transformation shown above? A. 1) NaNH2 2) CH3CH2OH B. O2
The Correct Answer and Explanation is:
To efficiently synthesize propanoic acid (CH₃CH₂COOH) from acetylene (HC≡CH), we need to perform a series of reactions that build a 3-carbon carboxylic acid from a 2-carbon alkyne. Let’s walk through the correct synthetic route:
Step-by-step Reaction Pathway:
- Start with acetylene (HC≡CH)
Acetylene has two sp-hybridized carbons and serves as a 2-carbon building block. - Step A: Alkylation with ethyl bromide (CH₃CH₂Br)
Use NaNH₂ to deprotonate acetylene, forming the acetylide anion (HC≡C⁻). Then add ethyl bromide (CH₃CH₂Br) to perform nucleophilic substitution, extending the carbon chain: HC≡CH→CH₃CH₂Br1.NaNH2HC≡CCH2CH3HC≡CH \xrightarrow[\text{CH₃CH₂Br}]{1. NaNH₂} HC≡CCH₂CH₃HC≡CH1.NaNH2CH₃CH₂BrHC≡CCH2CH3 This gives 1-butyne. - Step B: Oxidative cleavage to carboxylic acid
Oxidize 1-butyne with O₃ (ozone) followed by oxidative work-up (e.g., H₂O₂) to cleave the triple bond and form carboxylic acids: HC≡CCH2CH3→1. O₃, 2. H₂O₂CH3CH2COOHHC≡CCH₂CH₃ \xrightarrow{\text{1. O₃, 2. H₂O₂}} CH₃CH₂COOHHC≡CCH2CH31. O₃, 2. H₂O₂CH3CH2COOH This yields propanoic acid as the final product.
Structure of Intermediate X:
The intermediate X is HC≡CCH₂CH₃ (1-butyne), not ethene (H₂C=CH₂) as shown in the incorrect student response.
Explanation
The transformation of acetylene into propanoic acid is a classic carbon-chain extension followed by oxidation. Acetylene (HC≡CH) is a highly acidic alkyne, and treatment with a strong base like NaNH₂ generates an acetylide anion. This nucleophilic species is ideal for SN2 alkylation with a primary alkyl halide such as ethyl bromide (CH₃CH₂Br), forming 1-butyne (HC≡CCH₂CH₃).
The next critical step is the oxidative cleavage of the carbon-carbon triple bond. Ozonolysis, particularly with a peroxide work-up (like H₂O₂), cleaves alkynes into two carboxylic acid groups. Since the triple bond is internal (not terminal), the reaction produces two acids. For 1-butyne, cleavage gives propanoic acid and formic acid, but under conditions aimed specifically at the terminal portion, propanoic acid is the major product of interest.
The incorrect structure of X (ethene, H₂C=CH₂) indicates a misunderstanding—perhaps confusing a double bond product instead of the required triple-bonded intermediate. Ethene cannot be converted directly into propanoic acid via this method.
Thus, the correct reagent sequence is:
- A. 1) NaNH₂ 2) CH₃CH₂Br
- B. O₃/H₂O₂
This method efficiently builds the 3-carbon chain and oxidizes it to the desired propanoic acid.
