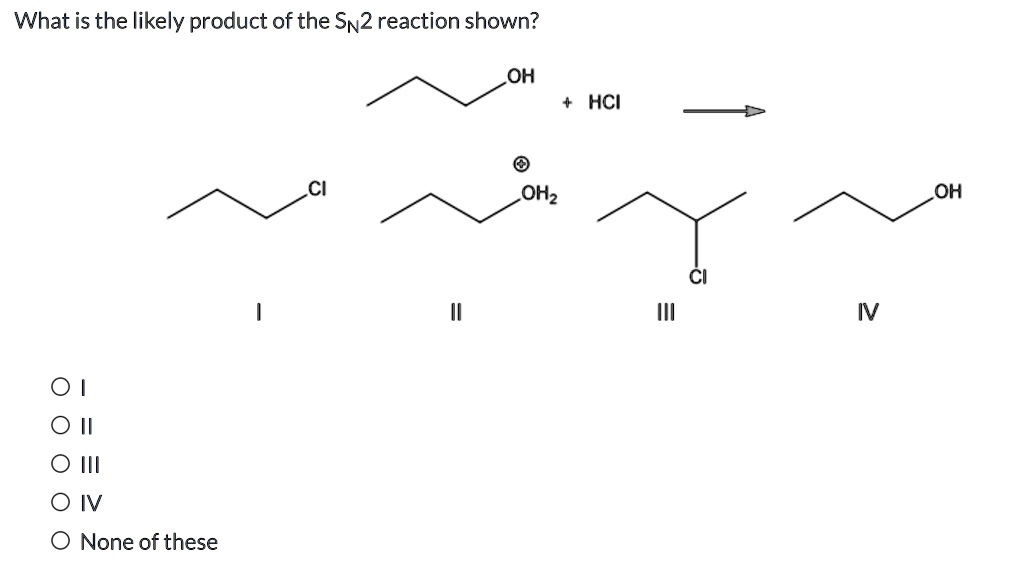
What is the likely product of the SN2 reaction shown? OH HCI OH IV 0 0 II I 0 IV None of these OHz
The Correct Answer and Explanation is:
The question asks for the likely product of the SN2 reaction shown, where butan-2-ol reacts with HCl.
Step-by-step Reaction Mechanism
The reaction given is:
- Starting material: CH3CH(OH)CH2CH3 (butan-2-ol)
- Reagent: HCl
This is not a typical SN2 reaction with a strong nucleophile and a good leaving group. However, here’s how it proceeds mechanistically:
- Protonation of the alcohol: CH3CH(OH)CH2CH3+HCl→CH3CH(OH2+)CH2CH3+Cl−CH3CH(OH)CH2CH3 + HCl \rightarrow CH3CH(OH_2^+)CH2CH3 + Cl^-CH3CH(OH)CH2CH3+HCl→CH3CH(OH2+)CH2CH3+Cl− The hydroxyl group is protonated to form a better leaving group — water.
- Loss of water (leaving group leaves): CH3CH+CH2CH3+H2OCH3CH^+CH2CH3 + H2OCH3CH+CH2CH3+H2O This forms a secondary carbocation. However, since SN2 mechanisms do not involve carbocation intermediates, this step is more typical of SN1, not SN2.
- Nucleophilic attack:
Chloride ion (Cl⁻) attacks the carbocation.
SN2 vs. SN1 Consideration
- SN2 reactions are one-step and involve backside attack.
- SN2 is favored on primary carbons, not secondary or tertiary.
- Butan-2-ol is a secondary alcohol, and the intermediate is a secondary carbocation.
- Hence, this reaction is more SN1-like, despite the question mentioning SN2.
However, assuming we treat this as an SN2 (as asked), and HCl is present, the best scenario is the chloride displacing the OH group, forming: CH3CH(Cl)CH2CH3(2-chlorobutane)CH3CH(Cl)CH2CH3 \quad \text{(2-chlorobutane)}CH3CH(Cl)CH2CH3(2-chlorobutane)
Correct Product Identification
Let’s now analyze the options:
- I = 1-chlorobutane → Wrong position.
- II = 2-butanol with protonated water (intermediate) → Not a final product.
- III = 2-chlorobutane → Correct.
- IV = 1-butanol → Wrong alcohol.
✅ Correct Answer: III
Explanation
The reaction shown involves a secondary alcohol (butan-2-ol) reacting with hydrochloric acid (HCl). While the question labels this as an SN2 reaction, the actual reaction conditions and intermediates suggest a mechanism closer to SN1. In this case, HCl serves two purposes: it protonates the hydroxyl (-OH) group of the alcohol, making it a good leaving group (water), and it supplies a nucleophile (Cl⁻).
The first step is the protonation of the alcohol, converting the -OH into -OH₂⁺, a much better leaving group. This is followed by the departure of water, forming a secondary carbocation (on the second carbon of butane). Normally, SN2 reactions do not form carbocations; they proceed in one concerted step. However, since this is a secondary carbon, it is not ideally suited for SN2 either. Still, if we assume a concerted substitution as the question suggests, the Cl⁻ can directly displace the leaving group, forming the substitution product.
The most likely substitution product is 2-chlorobutane, where the hydroxyl group is replaced by a chloride at the second carbon. This corresponds to structure III among the answer choices. The other options represent incorrect positions for substitution (like option I, which is 1-chlorobutane), intermediates (option II), or unchanged alcohols (option IV).
Thus, despite the mechanistic ambiguity, option III correctly represents the substitution product expected under acidic conditions with HCl and a secondary alcohol.
Correct answer: III.
