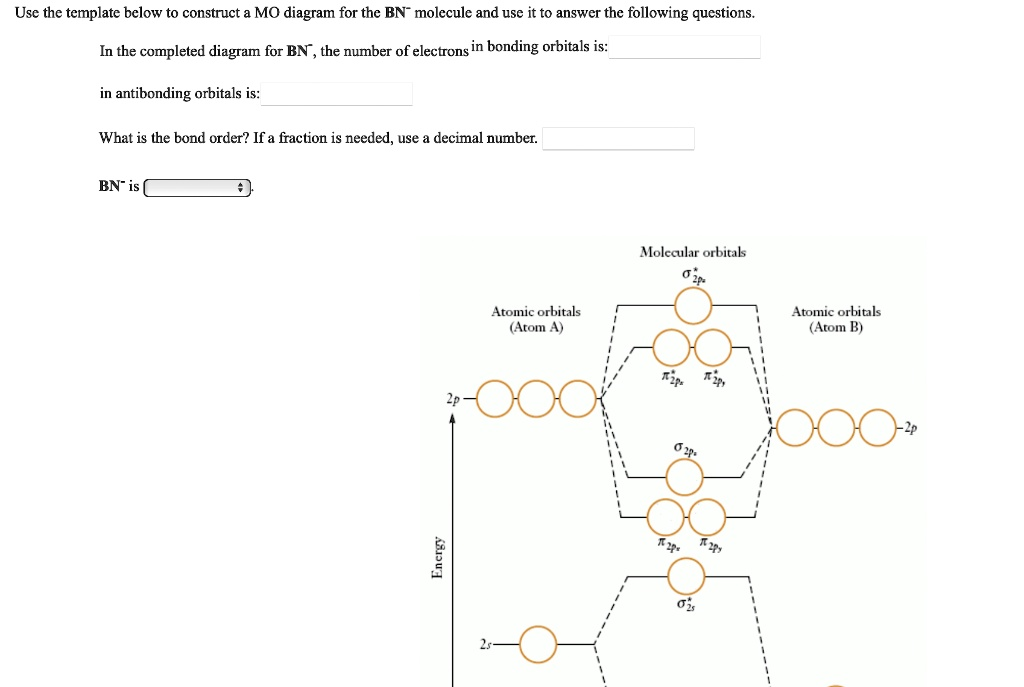
Use the template below to construct a MO diagram for the BN
molecule and use it to answer the following questions. In the completed diagram for BN
, the number of electrons in bonding orbitals is: in antibonding orbitals is: What is the bond order? If a fraction is needed, use a decimal number. BN
is Energy Atomic orbitals (Atom A) Molecular orbitals
Atomic orbitals (Atom B)
The Correct Answer and Explanation is:
To solve the problem and construct the MO (molecular orbital) diagram for the BN⁻ ion, we first need to consider the total number of electrons in the BN⁻ molecule.
Step 1: Determine Total Electrons
- Boron (B) has 5 electrons
- Nitrogen (N) has 7 electrons
- The negative charge (⁻) adds 1 extra electron
Total electrons = 5 (B) + 7 (N) + 1 (extra) = 13 electrons
Step 2: Use the Correct MO Energy Ordering
Since BN is a heteronuclear diatomic molecule and relatively light (similar to molecules like B₂, C₂, N₂), it follows the MO ordering:
scssCopyEditσ(2s), σ*(2s), π(2p), σ(2p), π*(2p), σ*(2p)
Step 3: Fill the Molecular Orbitals with Electrons
We now fill 13 electrons in order of increasing energy:
- σ(2s) → 2 electrons (bonding)
- σ(2s)* → 2 electrons (antibonding)
- π(2p) → 4 electrons (2 in each π orbital, bonding)
- σ(2p) → 2 electrons (bonding)
- π(2p)* → 2 electrons (antibonding)
- σ(2p)* → 1 electron (antibonding)
Step 4: Count Bonding and Antibonding Electrons
- Bonding electrons:
σ(2s) = 2
π(2p) = 4
σ(2p) = 2
Total = 8 - Antibonding electrons:
σ*(2s) = 2
π*(2p) = 2
σ*(2p) = 1
Total = 5
Step 5: Calculate Bond Order
Bond Order=(Bonding electrons−Antibonding electrons)2=8−52=32=1.5\text{Bond Order} = \frac{(\text{Bonding electrons} – \text{Antibonding electrons})}{2} = \frac{8 – 5}{2} = \frac{3}{2} = 1.5Bond Order=2(Bonding electrons−Antibonding electrons)=28−5=23=1.5
Step 6: Determine Magnetic Behavior
- Since BN⁻ has one unpaired electron (in the σ*2p orbital), it is paramagnetic.
✅ Final Answers
- Bonding electrons: 8
- Antibonding electrons: 5
- Bond order: 1.5
- BN⁻ is: Paramagnetic
📘 Explanation (Summary)
In molecular orbital (MO) theory, atomic orbitals combine to form bonding and antibonding molecular orbitals. For BN⁻, the molecular orbitals are filled based on energy levels, following the Aufbau principle and Hund’s rule. Because it’s a light heteronuclear molecule, the MO energy order resembles that of B₂ and C₂. By distributing 13 electrons across these orbitals, we determine that 8 are bonding and 5 are antibonding. Using the bond order formula, we calculate a bond order of 1.5, suggesting moderate bond strength between B and N. The presence of an unpaired electron also makes BN⁻ paramagnetic.
