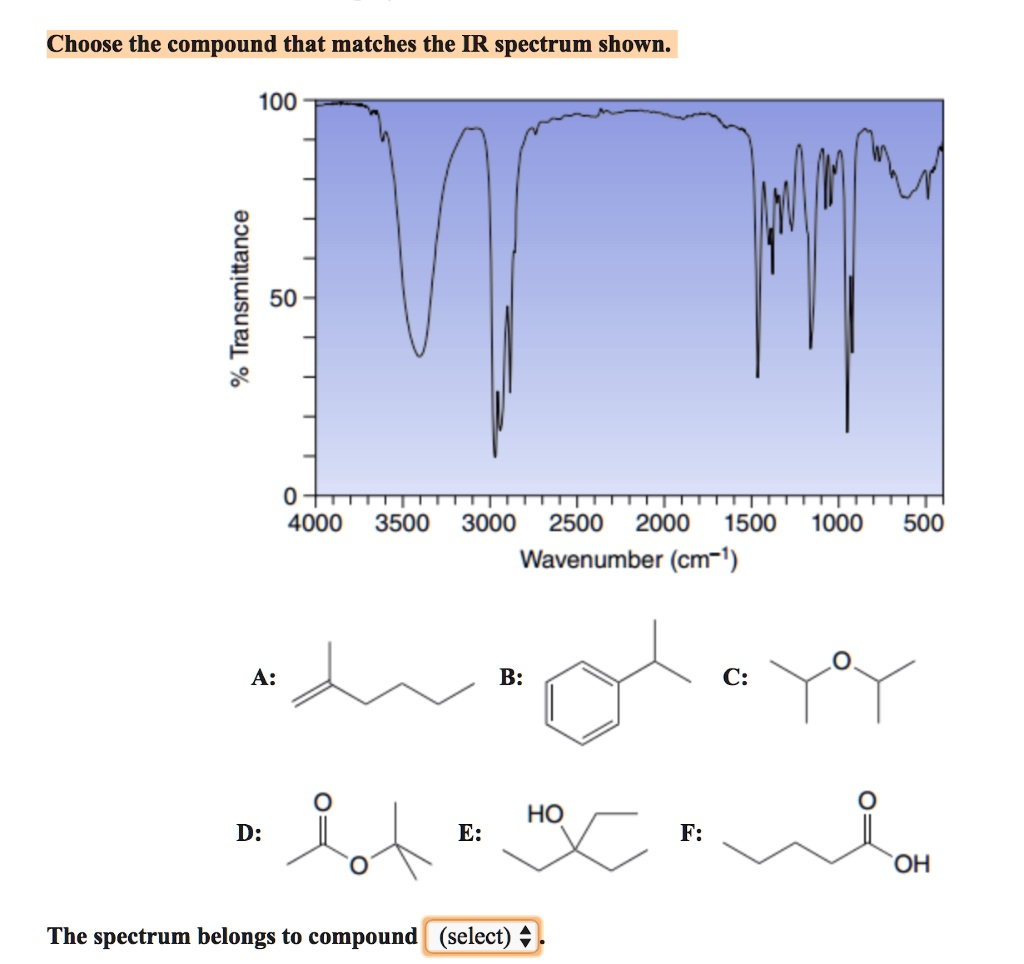
Choose the compound that matches the IR spectrum shown: 100 = Kw L 50 68 0 4000 3500 3000 2500 2000 1500 Wavenumber (cmâ»Â¹) 1000 500 A: B: HO D: E: F: OH The spectrum belongs to compound (select)
The Correct Answer and Explanation is:
An analysis of the provided IR spectrum reveals several key absorption bands that are crucial for identifying the unknown compound.
- O-H Stretch (Carboxylic Acid): The most prominent feature of this spectrum is the extremely broad and strong absorption band that dominates the region from approximately 3600 cm⁻¹ down to 2500 cm⁻¹. This exceptionally wide peak is the hallmark of the O-H stretching vibration in a hydrogen-bonded carboxylic acid dimer. The broadness is caused by the extensive hydrogen bonding between two acid molecules. This single feature strongly suggests the presence of a –COOH group.
- C=O Stretch (Carbonyl): There is a very strong and sharp absorption peak located at approximately 1710 cm⁻¹. This peak falls squarely in the carbonyl region (1650-1850 cm⁻¹) and is characteristic of the C=O stretch of a saturated carboxylic acid. The combination of this peak with the aforementioned broad O-H stretch is definitive evidence for a carboxylic acid functional group.
- C-H Stretch (Alkyl): The spectrum shows several sharp peaks just below 3000 cm⁻¹ (in the range of 2850-2960 cm⁻¹). These are characteristic of C-H stretching vibrations from sp³-hybridized carbon atoms, indicating the presence of an alkyl (alkane) portion in the molecule.
Now let’s evaluate the given options:
- A (Alkene): Lacks both the O-H and C=O groups.
- B (Aromatic hydrocarbon): Lacks both the O-H and C=O groups.
- C (Ether): Lacks both the O-H and C=O groups.
- D (Ester): Contains a C=O group but lacks the O-H group. An ester C=O stretch typically appears at a higher wavenumber (~1740 cm⁻¹).
- E (Alcohol): Contains an O-H group, which would show a broad peak around 3200-3600 cm⁻¹, but it would not be as exceptionally broad as the one observed. More importantly, it lacks a C=O group.
- F (Carboxylic Acid): This compound, pentanoic acid, possesses a carboxylic acid group (–COOH) and an alkyl chain. It would exhibit all the characteristic peaks seen in the spectrum: the very broad O-H stretch, the strong C=O stretch around 1710 cm⁻¹, and the sp³ C-H stretches below 3000 cm⁻¹.
Based on this analysis, the spectrum perfectly matches the expected absorptions for pentanoic acid.
The spectrum belongs to compound (F).
