Write a balanced chemical equation for the combustion of methane in oxygen. Be sure to include states of matter in your balanced equation. 2CH
(g) + 4O
(g)
2CO
(g) + 4H
O(g)
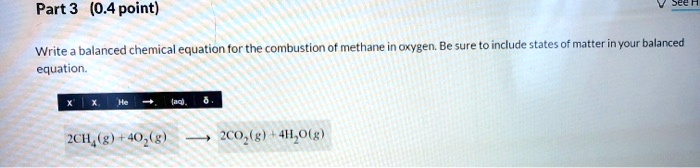
The Correct Answer and Explanation is:
Correct Balanced Chemical Equation: CH4(g)+2O2(g)→CO2(g)+2H2O(g)\text{CH}_4 (g) + 2 \text{O}_2 (g) \rightarrow \text{CO}_2 (g) + 2 \text{H}_2\text{O} (g)CH4(g)+2O2(g)→CO2(g)+2H2O(g)
Explanation:
Combustion reactions are chemical reactions where a substance reacts with oxygen to produce heat and light. In the case of methane (CH₄), which is a simple hydrocarbon and the main component of natural gas, combustion occurs when it reacts with oxygen (O₂) from the air.
The general form of a hydrocarbon combustion reaction is: Hydrocarbon+O2→CO2+H2O\text{Hydrocarbon} + \text{O}_2 \rightarrow \text{CO}_2 + \text{H}_2\text{O}Hydrocarbon+O2→CO2+H2O
For methane, the unbalanced equation starts as: CH4(g)+O2(g)→CO2(g)+H2O(g)\text{CH}_4 (g) + \text{O}_2 (g) \rightarrow \text{CO}_2 (g) + \text{H}_2\text{O} (g)CH4(g)+O2(g)→CO2(g)+H2O(g)
We now balance the equation by following these steps:
- Balance Carbon (C):
There is one carbon atom in CH₄ and one carbon atom in CO₂. Carbon is balanced. - Balance Hydrogen (H):
There are four hydrogen atoms in CH₄ and only two in one molecule of H₂O. To balance hydrogen, we place a coefficient of 2 in front of H₂O:
CH4(g)+O2(g)→CO2(g)+2H2O(g)\text{CH}_4 (g) + \text{O}_2 (g) \rightarrow \text{CO}_2 (g) + 2 \text{H}_2\text{O} (g)CH4(g)+O2(g)→CO2(g)+2H2O(g)
- Balance Oxygen (O):
On the right-hand side, there are two oxygen atoms from CO₂ and two from 2 H₂O (total 4 oxygen atoms). To get 4 oxygen atoms on the left-hand side, we place a coefficient of 2 in front of O₂:
CH4(g)+2O2(g)→CO2(g)+2H2O(g)\text{CH}_4 (g) + 2 \text{O}_2 (g) \rightarrow \text{CO}_2 (g) + 2 \text{H}_2\text{O} (g)CH4(g)+2O2(g)→CO2(g)+2H2O(g)
Now the equation is balanced with 1 carbon, 4 hydrogen, and 4 oxygen atoms on both sides. The states of matter indicate that methane, oxygen, carbon dioxide, and water vapor are all in the gaseous state during combustion.
This balanced equation shows that burning one mole of methane requires two moles of oxygen and produces one mole of carbon dioxide and two moles of water vapor.
