Given the parametric equations:
𝑥
1
−
𝑡
2
,
𝑦
1
+
𝑡
x=1−t
2
,y=1+t
We are to find:
𝑑
𝑦
𝑑
𝑥
dx
dy
— the first derivative of
𝑦
y with respect to
𝑥
x,
𝑑
2
𝑦
𝑑
𝑥
2
dx
2
d
2
y
— the second derivative of
𝑦
y with respect to
𝑥
x,
without eliminating the parameter.
Step 1: First Derivative
𝑑
𝑦
𝑑
𝑥
dx
dy
Using parametric differentiation, the first derivative is given by:
𝑑
𝑦
𝑑
𝑥
𝑑
𝑦
𝑑
𝑡
𝑑
𝑥
𝑑
𝑡
dx
dy
=
dt
dx
dt
dy
Differentiate
𝑥
x and
𝑦
y with respect to
𝑡
t:
𝑑
𝑥
𝑑
𝑡
𝑑
𝑑
𝑡
(
1
−
𝑡
2
)
−
2
𝑡
dt
dx
=
dt
d
(1−t
2
)=−2t
𝑑
𝑦
𝑑
𝑡
𝑑
𝑑
𝑡
(
1
+
𝑡
)
1
dt
dy
=
dt
d
(1+t)=1
So,
𝑑
𝑦
𝑑
𝑥
1
−
2
𝑡
−
1
2
𝑡
dx
dy
=
−2t
1
=−
2t
1
Step 2: Second Derivative
𝑑
2
𝑦
𝑑
𝑥
2
dx
2
d
2
y
The second derivative in parametric form is given by:
𝑑
2
𝑦
𝑑
𝑥
2
𝑑
𝑑
𝑡
(
𝑑
𝑦
𝑑
𝑥
)
÷
𝑑
𝑥
𝑑
𝑡
dx
2
d
2
y
=
dt
d
(
dx
dy
)÷
dt
dx
We already have
𝑑
𝑦
𝑑
𝑥
−
1
2
𝑡
dx
dy
=−
2t
1
, so compute its derivative with respect to
𝑡
t:
𝑑
𝑑
𝑡
(
−
1
2
𝑡
)
1
2
𝑡
2
dt
d
(−
2t
1
)=
2t
2
1
Now divide by
𝑑
𝑥
𝑑
𝑡
−
2
𝑡
dt
dx
=−2t:
𝑑
2
𝑦
𝑑
𝑥
2
1
2
𝑡
2
−
2
𝑡
−
1
4
𝑡
3
dx
2
d
2
y
=
−2t
2t
2
1
=−
4t
3
1
Final Answers:
𝑑
𝑦
𝑑
𝑥
−
1
2
𝑡
,
𝑑
2
𝑦
𝑑
𝑥
2
−
1
4
𝑡
3
dx
dy
=−
2t
1
,
dx
2
d
2
y
=−
4t
3
1
Explanation (300 words):
When working with parametric equations, such as
𝑥
1
−
𝑡
2
x=1−t
2
and
𝑦
1
+
𝑡
y=1+t, we treat both
𝑥
x and
𝑦
y as functions of a third variable
𝑡
t, known as the parameter. Instead of expressing
𝑦
y directly in terms of
𝑥
x, we differentiate each with respect to
𝑡
t, and use these derivatives to find the derivatives of
𝑦
y with respect to
𝑥
x.
To find the first derivative
𝑑
𝑦
𝑑
𝑥
dx
dy
, we apply the chain rule of calculus. Since
𝑑
𝑦
/
𝑑
𝑥
(
𝑑
𝑦
/
𝑑
𝑡
)
/
(
𝑑
𝑥
/
𝑑
𝑡
)
dy/dx=(dy/dt)/(dx/dt), we compute the derivatives:
𝑑
𝑦
/
𝑑
𝑡
1
dy/dt=1, since
𝑦
1
+
𝑡
y=1+t,
𝑑
𝑥
/
𝑑
𝑡
−
2
𝑡
dx/dt=−2t, because
𝑥
1
−
𝑡
2
x=1−t
2
.
Substituting, we get
𝑑
𝑦
𝑑
𝑥
1
−
2
𝑡
−
1
2
𝑡
dx
dy
=
−2t
1
=−
2t
1
.
To find the second derivative
𝑑
2
𝑦
𝑑
𝑥
2
dx
2
d
2
y
, we use the formula:
𝑑
2
𝑦
𝑑
𝑥
2
𝑑
𝑑
𝑡
(
𝑑
𝑦
𝑑
𝑥
)
÷
𝑑
𝑥
𝑑
𝑡
dx
2
d
2
y
=
dt
d
(
dx
dy
)÷
dt
dx
This involves differentiating
−
1
2
𝑡
−
2t
1
with respect to
𝑡
t, which gives
1
2
𝑡
2
2t
2
1
, and then dividing this result by
−
2
𝑡
−2t, the derivative of
𝑥
x. Thus:
𝑑
2
𝑦
𝑑
𝑥
2
1
2
𝑡
2
−
2
𝑡
−
1
4
𝑡
3
dx
2
d
2
y
=
−2t
2t
2
1
=−
4t
3
1
This process allows us to compute the derivatives directly from the parametric equations without needing to eliminate the parameter.
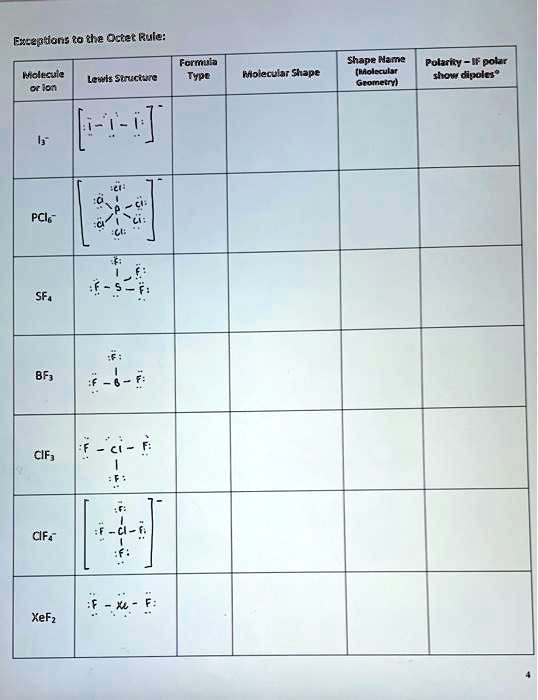
The Correct Answer and Explanation is:
Completed Chemistry Worksheet: Exceptions to the Octet Rule
| Molecule or Ion | Lewis Structure | Formula Type | Molecular Shape (Description) | Shape Name (Molecular Geometry) | Polarity – if polar show dipoles |
| I₃⁻ | [ :Ï — Ï — Ï: ]⁻ (Central I has 3 lone pairs) | AX₂E₃ | The central Iodine atom is bonded to two other Iodine atoms in a straight line, with three lone pairs arranged in a plane around the central atom’s equator. | Linear | Nonpolar (The symmetrical arrangement of atoms causes the dipoles to cancel.) |
| PCl₆⁻ | [P bonded to 6 Cl]⁻ (P has no lone pairs) | AX₆ | A central Phosphorus atom is bonded to six Chlorine atoms, with four in a square plane and one above and one below the plane. | Octahedral | Nonpolar (The symmetrical octahedral geometry causes all bond dipoles to cancel out.) |
| SF₄ | S bonded to 4 F (S has one lone pair) | AX₄E₁ | A central Sulfur atom is bonded to four Fluorine atoms. The lone pair on the sulfur atom pushes the bonds away, creating a shape that resembles a seesaw. | Seesaw | Polar (The shape is asymmetrical due to the lone pair. There is a net dipole moment pointing towards the fluorine atoms.) |
| BF₃ | B bonded to 3 F (B has an incomplete octet) | AX₃ | A central Boron atom is bonded to three Fluorine atoms, all lying in the same plane and spaced 120° apart. | Trigonal Planar | Nonpolar (The symmetrical arrangement of the B-F bonds in a plane causes the dipoles to cancel.) |
| ClF₃ | Cl bonded to 3 F (Cl has two lone pairs) | AX₃E₂ | A central Chlorine atom is bonded to three Fluorine atoms. Two lone pairs on the chlorine push the bonds into a T-shape. | T-shaped | Polar (The shape is asymmetrical. The bond dipoles do not cancel, resulting in a net dipole moment along the stem of the ‘T’.) |
| ClF₄⁻ | [Cl bonded to 4 F]⁻ (Cl has two lone pairs) | AX₄E₂ | A central Chlorine atom is bonded to four Fluorine atoms that lie in a single plane. Two lone pairs are located above and below this plane. | Square Planar | Nonpolar (Although the bonds are polar, the symmetrical arrangement of the four Fluorine atoms around the central Chlorine causes their dipoles to cancel out.) |
| XeF₂ | Xe bonded to 2 F (Xe has three lone pairs) | AX₂E₃ | A central Xenon atom is bonded to two Fluorine atoms. Three lone pairs on the Xenon atom are arranged in a plane, forcing the Fluorine atoms to opposite sides. | Linear | Nonpolar (The two Xe-F bonds are on opposite sides of the central atom, so their dipoles are equal and opposite, and they cancel out.) |
thumb_upthumb_down
