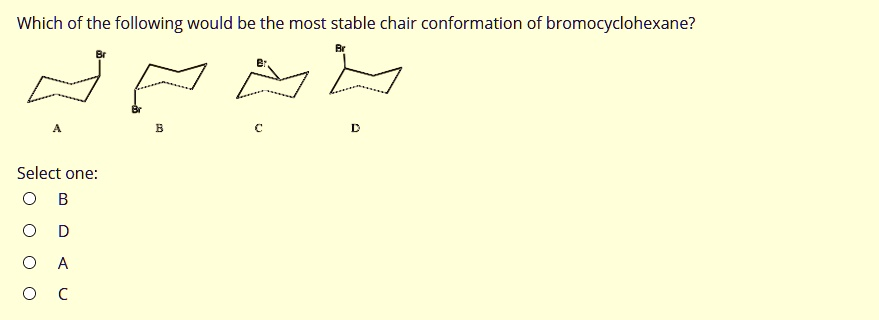
Which of the following would be the most stable chair conformation of bromocyclohexane? Select one:
The Correct Answer and Explanation is:
Correct Answer: A
Explanation:
The stability of a substituted cyclohexane in its chair conformation is primarily determined by the position of the substituent group on the ring. The cyclohexane ring can adopt a low-energy, non-planar “chair” conformation that minimizes both angle strain and torsional strain. In this conformation, there are two distinct types of positions for substituents: axial and equatorial.
- Axial vs. Equatorial Positions:
- Axial positions are parallel to the principal axis of symmetry of the ring, pointing either straight up or straight down.
- Equatorial positions point outwards from the “equator” of the ring, roughly within the general plane of the carbon atoms.
- Steric Strain and 1,3-Diaxial Interactions:
The key factor determining the stability of a conformation is steric strain. When a substituent larger than hydrogen is in an axial position, it experiences significant steric repulsion from the other two axial hydrogen atoms located on the same side of the ring. These interactions occur between the substituent at carbon-1 and the axial hydrogens at carbon-3 and carbon-5, and are known as 1,3-diaxial interactions. This repulsion increases the overall energy of the conformation, making it less stable.Conversely, a substituent in the equatorial position points away from the bulk of the ring, minimizing steric interactions. It does not experience significant 1,3-diaxial strain. - Analysis of Bromocyclohexane:
For bromocyclohexane, the substituent is a bromine (Br) atom. Bromine is considerably larger than a hydrogen atom. To achieve the most stable, lowest-energy conformation, the bulky bromine atom must occupy the position that minimizes steric strain. Therefore, the chair conformation with the bromine atom in the equatorial position is overwhelmingly favored over the conformation with the bromine in the axial position. - Evaluating the Options:
- Structure A: The bromine atom is in an equatorial position, pointing away from the ring. This is a low-energy, stable conformation.
- Structure B: The bromine atom is in an axial position, pointing straight down. It would experience 1,3-diaxial interactions with the axial hydrogens, making this conformation unstable.
- Structure C: The bromine atom is in an axial position, pointing straight up. Similar to B, this conformation is destabilized by 1,3-diaxial interactions.
- Structure D: The bromine atom is also shown in an equatorial position on a different chair representation. Like A, this is a stable conformation.
Since the question asks for the most stable chair conformation, we must choose the structure where the bromine atom is in the equatorial position. Both A and D depict this stable arrangement. Given the choices, Structure A is a correct representation of the most stable conformation of bromocyclohexane.
