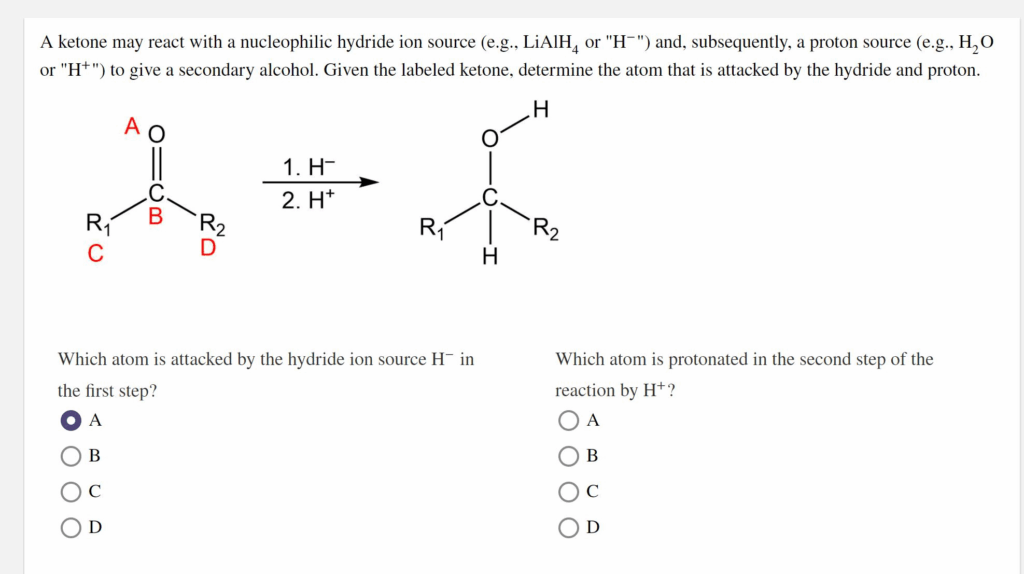
A ketone may react with a nucleophilic hydride ion source (e.g.,
or “
“) and, subsequently, a proton source (e.g.,
or “
“) to give a secondary alcohol. Given the labeled ketone, determine the atom that is attacked by the hydride and proton. Which atom is attacked by the hydride ion source
in
Which atom is protonated in the second step of the the first step? reaction by
?
A B
D
Here are the correct answers to the questions, followed by a detailed explanation of the reaction mechanism.
Correct Answers:
- Which atom is attacked by the hydride ion source H⁻ in the first step?
- Correct Answer: B
- Which atom is protonated in the second step of the reaction by H⁺?
- Correct Answer: A
Explanation of the Reaction Mechanism
The reaction shown is the nucleophilic addition of a hydride ion to a ketone, resulting in the formation of a secondary alcohol. This is a fundamental reaction in organic chemistry, often referred to as the reduction of a ketone. The process occurs in two distinct steps.
Step 1: Nucleophilic Attack by the Hydride Ion
The first step of the reaction involves the attack of the nucleophilic hydride ion (H⁻). To understand where the hydride will attack, we must first analyze the electronic structure of the ketone’s carbonyl group (C=O).
- Polarity of the Carbonyl Group: The oxygen atom (labeled A) is significantly more electronegative than the carbon atom (labeled B). This difference in electronegativity causes the C=O double bond to be highly polarized. The electron density is pulled towards the oxygen, giving it a partial negative charge (δ⁻). Consequently, the carbonyl carbon is left with a partial positive charge (δ⁺), making it electrophilic (electron-seeking).
- The Nucleophile and Electrophile: The hydride ion (H⁻) is an electron-rich species, making it a strong nucleophile (nucleus-seeking). In chemical reactions, nucleophiles are attracted to and attack electrophiles.
- The Attack: Therefore, the nucleophilic hydride ion (H⁻) will attack the electron-deficient carbonyl carbon, atom B. As the new C-H bond forms, the weaker pi (π) bond of the C=O double bond breaks, and the pair of electrons moves onto the electronegative oxygen atom (A). This step results in the formation of a tetrahedral intermediate called an alkoxide ion.
Step 2: Protonation of the Alkoxide Intermediate
The second step is an acid-base reaction that completes the formation of the alcohol.
- The Alkoxide Intermediate: After the first step, the oxygen atom (A) now bears a full negative charge, making the alkoxide ion a strong base.
- Protonation: When a proton source (H⁺), such as water (H₂O) or dilute acid, is added to the reaction mixture, the negatively charged oxygen atom (A) acts as a base and readily accepts a proton.
- Final Product: This protonation step neutralizes the intermediate, forming the final, stable secondary alcohol product. Thus, atom A is the site of protonation in the second step of the reaction.
