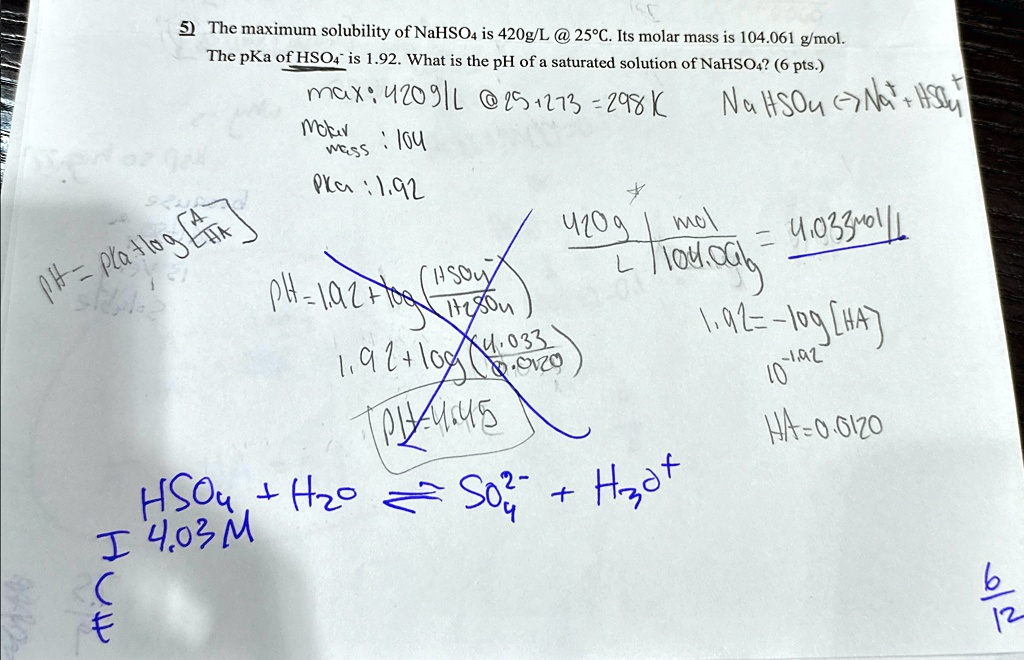
Based on the red ink corrections, solve. The maximum solubility of NaHSO4 is 420 g/L @ 25°C. Its molar mass is 104.061 g/mol. The pKa of HSO4^- is 1.92. What is the pH of a saturated solution of NaHSO4? (6 pts.) Plan: 1.92 The maximum solubility of NaHSO4 is 420 g/L @ 25°C. Its molar mass is 104.061 g/mol. The pKa of HSO4^- is 1.92. What is the pH of a saturated solution of NaHSO4? (6 pts.) max: 420 g/L @ 25°C Prc: 1.92 420 g/mol x 1 mol/104.061 g = 4.033 mol/L SO4^-2 + H2O -> HSO4^- + OH^- 1.92 = -log([HSO4^-]/[SO4^-2]) 1.92 = -log(4.033/x) x = 0.033 M pH = 1/2(pKa - log([A-]/[HA])) pH = 1/2(1.92 - log(0.033/4.033)) pH = 1/2(1.92 - 1.39) pH = 0.265
The Correct Answer and Explanation is:
Based on the provided information, the correct solution is as follows:
Correct Answer: The pH of the saturated solution is 0.67.
Explanation
This problem requires determining the pH of a saturated solution of sodium bisulfate (NaHSO₄), which involves a weak acid equilibrium calculation.
1. Calculate the Molar Concentration of the Saturated Solution:
First, the initial concentration of NaHSO₄ in the saturated solution is determined by converting the given solubility from grams per liter (g/L) to moles per liter (mol/L or M).
- Solubility = 420 g/L
- Molar Mass of NaHSO₄ = 104.061 g/mol
Molarity = (420 g/L) / (104.061 g/mol ) = 4.036 M NaHSO₄
2. Determine the Initial Concentration of the Acidic Ion:
Sodium bisulfate is an ionic salt that dissociates completely in water into sodium ions (Na⁺) and bisulfate ions (HSO₄⁻). The sodium ion is a spectator ion and does not affect the pH. The bisulfate ion, however, is a weak acid.
NaHSO₄(aq) → Na⁺(aq) + HSO₄⁻(aq)
Due to the 1:1 stoichiometry, the initial concentration of the bisulfate ion, [HSO₄⁻], is equal to the molarity of the solution, which is 4.036 M.
3. Set Up the Weak Acid Equilibrium:
The bisulfate ion (HSO₄⁻) acts as a weak acid, donating a proton to water to establish an equilibrium with sulfate ions (SO₄²⁻) and hydronium ions (H₃O⁺).
The equilibrium reaction is:
HSO₄⁻(aq) + H₂O(l) ⇌ SO₄²⁻(aq) + H₃O⁺(aq)
An ICE (Initial, Change, Equilibrium) table is used to find the equilibrium concentrations:
| Species | Initial (M) | Change (M) | Equilibrium (M) |
| HSO₄⁻ | 4.036 | -x | 4.036 – x |
| SO₄²⁻ | 0 | +x | x |
| H₃O⁺ | 0 | +x | x |
4. Solve for the Hydronium Ion Concentration [H₃O⁺]:
The acid dissociation constant, Ka, is calculated from the given pKa:
- pKa = 1.92
- Ka = 10⁻ᵖᴷᵃ = 10⁻¹⁹² = 0.0120
The equilibrium expression is:
Ka = [SO₄²⁻][H₃O⁺] / [HSO₄⁻]
0.0120 = (x)(x) / (4.036 – x)
Because the initial concentration (4.036 M) is not significantly larger than the Ka value (the ratio C/Ka is less than 400), the simplifying assumption that ‘x’ is negligible cannot be used. The full quadratic equation must be solved:
x² = 0.0120 * (4.036 – x)
x² = 0.04843 – 0.0120x
x² + 0.0120x – 0.04843 = 0
Using the quadratic formula, x = [-b ± √(b²-4ac)] / 2a:
x = [-0.0120 ± √((0.0120)² – 4(1)(-0.04843))] / 2
x = [-0.0120 ± √(0.000144 + 0.19372)] / 2
x = [-0.0120 ± √(0.19386)] / 2
x = [-0.0120 ± 0.4403] / 2
Since concentration cannot be negative, the positive root is taken:
x = (0.4283) / 2 = 0.214 M
Thus, the equilibrium concentration of hydronium ions is [H₃O⁺] = 0.214 M.
5. Calculate the pH:
The pH is the negative logarithm of the hydronium ion concentration.
pH = -log[H₃O⁺]
pH = -log(0.214)
pH ≈ 0.67thumb_upthumb_down
