OsO4, NMO 2. NaHSO3, H2O Mg, THF Review Examples Vilika Reagents Select a Reagent
OsO4, NMO 2. NaHSO3, H2O Mg, THF Review Examples Vilika Reagents Select a Reagent
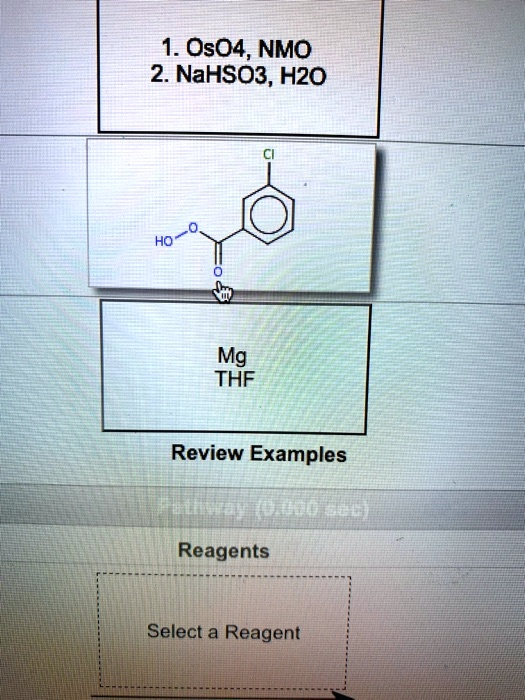
The Correct Answer and Explanation is:
The correct answer identifies the central molecule and explains its function as a reagent in organic chemistry.
Reagent Identification: meta-Chloroperoxybenzoic Acid (m-CPBA)
The chemical structure shown in the central box is meta-chloroperoxybenzoic acid, a reagent commonly known by its abbreviation m-CPBA. It is a type of peroxy acid, characterized by the -C(=O)OOH functional group, which makes it a powerful and selective oxidizing agent.
Primary Reaction: Epoxidation of Alkenes
The principal application of m-CPBA in organic synthesis is the epoxidation of alkenes. This reaction involves the direct transfer of an oxygen atom from the peroxy acid to the carbon-carbon double bond of an alkene, forming a three-membered cyclic ether called an epoxide (or oxirane). The other product of the reaction is meta-chlorobenzoic acid.
Mechanism and Stereochemistry:
The epoxidation reaction with m-CPBA proceeds through a concerted mechanism, often referred to as the “butterfly mechanism.” In a single, simultaneous step, the alkene’s π bond attacks the terminal, electrophilic oxygen of the peroxy acid, while the weak O-O bond breaks. This process occurs through a cyclic transition state, leading to the formation of the epoxide ring.
A crucial feature of this mechanism is its stereospecificity. The stereochemistry of the starting alkene is preserved in the epoxide product. This means that a cis-alkene will always yield a cis-epoxide (where the substituents are on the same side of the epoxide ring), and a trans-alkene will produce a trans-epoxide. This high degree of stereochemical control makes m-CPBA an invaluable tool for synthetic chemists.
Context with Other Displayed Reagents
The image also displays other common reagent systems, each for a distinct transformation:
- 1. OsO4, NMO; 2. NaHSO3, H2O: This is the reagent set for syn-dihydroxylation. It converts an alkene into a cis-1,2-diol by adding two hydroxyl (-OH) groups to the same face of the double bond.
- Mg, THF: These reagents are used to form a Grignard reagent from an alkyl or aryl halide. This reaction creates a potent carbon nucleophile (R-MgX) essential for forming new carbon-carbon bonds.
