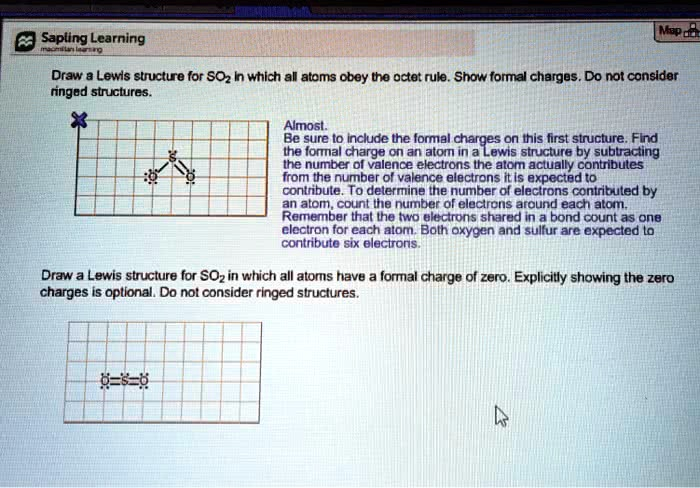
Sapling Learning macmillan learning Map Draw a Lewis structure for SO
in which all atoms obey the octet rule. Show formal charges. Do not consider ringed structures. Almost. Be sure to include the formal charges on this first structure. Find the formal charge on an atom in a Lewis structure by subtracting the number of valence electrons the atom actually contributes from the number of valence electrons it is expected to contribute. To determine the number of electrons contributed by an atom, count the number of electrons around each atom. Remember that the two electrons shared in a bond count as one electron for each atom. Both oxygen and sulfur are expected to contribute six electrons. Draw a Lewis structure for SO
in which all atoms have a formal charge of zero. Explicitly showing the zero charges is optional. Do not consider ringed structures. 0=S=0
The Correct Answer and Explanation is:
Of course. Here are the correct Lewis structures for sulfur dioxide (SO₂) based on the two different criteria, along with a detailed explanation.
Correct Lewis Structures for SO₂
1. Lewis Structure for SO₂ Obeying the Octet Rule:
This structure involves resonance, meaning there are two equivalent ways to draw it. A single valid resonance structure is shown below, as requested.
.. +1 -1
:O=S—O:
¨ ¨content_copydownloadUse code
(Note: Each bond line represents 2 electrons. The dots represent non-bonding (lone pair) electrons. The formal charges are shown above the corresponding atoms.)
2. Lewis Structure for SO₂ with Zero Formal Charges:
This structure minimizes formal charges but requires the central sulfur atom to have an expanded octet.
..
:O=S=O:
¨content_copydownloadUse code
Explanation
Drawing a correct Lewis structure involves a systematic process of counting valence electrons and distributing them to satisfy chemical bonding rules. For sulfur dioxide (SO₂), two primary models are often considered, leading to two different “correct” structures depending on the rules being prioritized.
Step 1: Calculate Total Valence Electrons
First, determine the total number of valence electrons available for bonding. Both sulfur (S) and oxygen (O) are in Group 16 of the periodic table, so each atom contributes 6 valence electrons.
- Sulfur: 1 atom × 6 valence e⁻ = 6 e⁻
- Oxygen: 2 atoms × 6 valence e⁻ = 12 e⁻
- Total Valence Electrons = 6 + 12 = 18 e⁻
Structure 1: Prioritizing the Octet Rule
The octet rule states that atoms tend to bond in such a way that they each have eight electrons in their valence shell.
- Arrange Atoms: Sulfur is less electronegative than oxygen, so it is the central atom: O–S–O.
- Form Bonds: Initially, form single bonds between the atoms (O–S–O), using 4 of the 18 electrons.
- Distribute Remaining Electrons: Place the remaining 14 electrons as lone pairs, starting with the outer (terminal) oxygen atoms to satisfy their octets. Each oxygen gets 6 lone pair electrons. This uses 12 electrons. The final 2 electrons are placed on the central sulfur atom.
- Satisfy Central Atom’s Octet: At this point, each oxygen has an octet, but the sulfur atom only has 6 electrons (two lone pair electrons and two single bonds). To give sulfur an octet, a lone pair from one of the oxygen atoms is shared to form a double bond. This results in a structure with one single bond and one double bond.
Formal Charges for Structure 1:
The formal charge helps determine the most plausible Lewis structure. It is calculated as:
Formal Charge = (Valence e⁻) – (Lone Pair e⁻) – (½ × Bonding e⁻)
- Double-bonded Oxygen: 6 – 4 – ½(4) = 0
- Central Sulfur: 6 – 2 – ½(6) = +1
- Single-bonded Oxygen: 6 – 6 – ½(2) = -1
This structure fulfills the octet rule for all atoms but results in formal charges.
Structure 2: Prioritizing Minimized Formal Charges
A guiding principle for Lewis structures is that the structure with formal charges closest to zero is generally more stable.
To eliminate the +1 and -1 formal charges from the first structure, another lone pair is taken from the single-bonded oxygen (with the -1 charge) and used to form a second double bond with the sulfur atom. This results in a structure where the central sulfur is double-bonded to both oxygen atoms.
Formal Charges for Structure 2:
- Each Oxygen: 6 – 4 – ½(4) = 0
- Central Sulfur: 6 – 2 – ½(8) = 0
In this arrangement, all atoms have a formal charge of zero. However, the central sulfur atom now has 10 valence electrons (one lone pair and four bonds), which is an expanded octet. This is permissible because sulfur is in the third period and can accommodate more than eight electrons in its valence shell.
