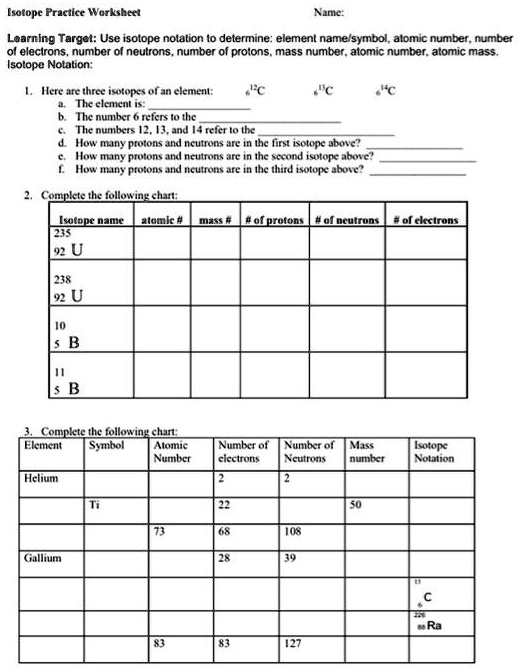
Isotope Practice Worksheet Name: Learning Target: Use isotope notation to determine: element name/symbol, atomic number, number of electrons, number of neutrons, number of protons, mass number, atomic number, atomic mass. Isotope Notation: 1. Here are three isotopes of an element: a. The element is:
b. The number 6 refers to the c. The numbers 12, 13, and 14 refer to the d. How many protons and neutrons are in the first isotope above? e. How many protons and neutrons are in the second isotope above? f. How many protons and neutrons are in the third isotope above? 2. Complete the following chart: Isotope name atomic # mass # # of protons # of neutrons # of electrons
- Complete the following chart: Element Symbol Atomic Number of Number of Mass Isotope Number electrons Neutrons number Notation Helium 2 2 Ti 22 50 73 68 108 Gallium 28 39 83 83 127
The Correct Answer and Explanation is:
Here is the completed worksheet with the correct answers.
Completed Isotope Practice Worksheet
1. Here are three isotopes of an element: ¹²C ¹³C ¹⁴C
a. The element is: Carbon
b. The number 6 refers to the atomic number
c. The numbers 12, 13, and 14 refer to the mass numbers
d. How many protons and neutrons are in the first isotope above? 6 protons, 6 neutrons
e. How many protons and neutrons are in the second isotope above? 6 protons, 7 neutrons
f. How many protons and neutrons are in the third isotope above? 6 protons, 8 neutrons
2. Complete the following chart:
| Isotope name | atomic # | mass # | # of protons | # of neutrons | # of electrons |
| Uranium-235 | 92 | 235 | 92 | 143 | 92 |
| Uranium-238 | 92 | 238 |
- The element is Carbon, as indicated by the symbol ‘C’.
- The number 6 (implied, as Carbon is always element 6) is the atomic number, which is the count of protons.
- The numbers 12, 13, and 14 are the respective mass numbers for each isotope.
- The number of neutrons is found by subtracting the atomic number from the mass number (Neutrons = A – Z).
- For ¹²C: 12 – 6 = 6 neutrons.
- For ¹³C: 13 – 6 = 7 neutrons.
- For ¹⁴C: 14 – 6 = 8 neutrons.
Charts 2 & 3 Walkthrough:
The charts are completed by applying the fundamental concepts. For any given row, you can deduce the missing information from the data provided.
- Finding Protons: The number of protons is always equal to the atomic number.
- Finding Electrons: In a neutral atom, the number of electrons is equal to the number of protons. If the electron count is different, it indicates an ion (e.g., Tantalum and Gallium rows).
- Finding Neutrons: Subtract the atomic number (protons) from the mass number.
- Finding Mass Number: Add the number of protons and neutrons together.
- Finding Element/Symbol: The atomic number uniquely identifies an element and its symbol on the periodic table. 92 | 146 | 92 |
| Boron-10 | 5 | 10 | 5 | 5 | 5 |
| Boron-11 | 5 | 11 | 5 | 6 | 5 |
3. Complete the following chart:
| Element | Symbol | Atomic Number | Number of electrons | Number of Neutrons | Mass number | Isotope Notation |
| Helium | He | 2 | 2 | 2 | 4 | ⁴₂He |
| Titanium | Ti | 22 | 22 | 28 | 50 | ⁵⁰₂₂Ti |
| Tantalum | Ta | 73 | 73 | 108 | 181 | ¹⁸¹₇₃Ta |
| Gallium | Ga | 31 | 31 | 39 | 70 | ⁷⁰₃₁Ga |
| Carbon | C | 6 | 6 | 7 | 13 | ¹³₆C |
| Radium | Ra | 88 | 88 | 138 | 226 | ²²⁶₈₈Ra |
| Bismuth | Bi | 83 | 83 | 127 | 210 | ²¹⁰₈₃Bi |
Explanation
The solutions to this worksheet are based on the fundamental principles of atomic structure and isotope notation. Each atom is defined by the number of its subatomic particles: protons, neutrons, and electrons.
An element’s identity is determined solely by its atomic number (Z), which
