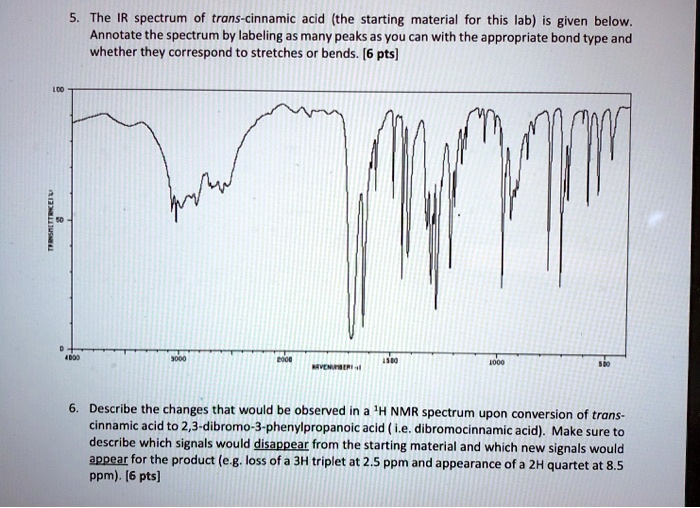
The IR spectrum of trans-cinnamic acid (the starting material for this lab) is given below. Annotate the spectrum by labeling as many peaks as you can with the appropriate bond type and whether they correspond to stretches or bends. [6 pts] 4000 3000 2000 1500 WAVENUMBER (cm-1) 1000 500 6. Describe the changes that would be observed in a
H NMR spectrum upon conversion of trans- cinnamic acid to 2,3-dibromo-3-phenylpropanoic acid (i.e. dibromocinnamic acid). Make sure to describe which signals would disappear from the starting material and which new signals would appear for the product (e.g. loss of a 3H triplet at 2.5 ppm and appearance of a 2H quartet at 8.5 ppm). [6 pts]
The Correct Answer and Explanation is:
Answer to Question 5: IR Spectrum Annotation
The provided IR spectrum is for trans-cinnamic acid (C₆H₅-CH=CH-COOH). The key absorption peaks are identified below:
- ~3300-2500 cm⁻¹ (very broad): This corresponds to the O-H stretch of the carboxylic acid functional group. The extreme broadness is due to strong intermolecular hydrogen bonding (dimerization).
- ~3060 cm⁻¹ (sharp, on top of the broad O-H): This peak represents the sp² C-H stretches. This includes both the vinylic (=C-H) and aromatic (Ar-H) C-H bonds.
- ~1685 cm⁻¹ (strong, sharp): This is the C=O stretch (carbonyl) of the carboxylic acid. Its frequency is lowered from the typical ~1710 cm⁻¹ because it is conjugated with both the alkene and the aromatic ring.
- ~1625 cm⁻¹ (medium): This absorption is from the C=C stretch of the alkene double bond.
- ~1580, 1495, 1450 cm⁻¹ (sharp): These peaks are characteristic of C=C in-ring stretches of the aromatic (phenyl) group.
- ~1310 cm⁻¹ (strong): This peak is attributed to the C-O stretch of the carboxylic acid group.
- ~980 cm⁻¹ (strong, sharp): This is a highly diagnostic peak for a trans-substituted alkene. It corresponds to the out-of-plane =C-H bend.
- ~765 cm⁻¹ and ~690 cm⁻¹ (strong): These two peaks are characteristic out-of-plane C-H bends for a monosubstituted benzene ring.
Answer to Question 6: ¹H NMR Spectrum Changes
The conversion of trans-cinnamic acid to 2,3-dibromo-3-phenylpropanoic acid involves the addition of Br₂ across the alkene double bond. This chemical transformation leads to significant and predictable changes in the ¹H NMR spectrum.
Signals that would disappear from the trans-cinnamic acid spectrum:
The most notable change is the disappearance of the signals from the two alkene protons (-CH=CH-). In the starting material, these appear as two distinct 1H doublets. One doublet is typically around 6.4 ppm and the other is further downfield around 7.7 ppm (often overlapping with the aromatic signals). A key feature of these doublets is their large coupling constant (J ≈ 12-18 Hz), which is characteristic of a trans configuration. These two signals will be completely absent in the product’s spectrum because the double bond is no longer present.
New signals that would appear for the 2,3-dibromo-3-phenylpropanoic acid product:
In place of the alkene signals, new signals corresponding to the two protons on the newly formed single bond (-CH(Br)-CH(Br)-) will emerge. These two protons are in different chemical environments and will couple to each other, resulting in two new 1H doublets. These signals will appear in the aliphatic region, typically between 4.5 and 5.5 ppm, due to the deshielding effect of the adjacent bromine atoms and other functional groups. The coupling constant for these new doublets will be much smaller (J ≈ 2-10 Hz) than the one observed for the trans-alkene protons.
The signals for the 5H aromatic protons (multiplet, ~7.4 ppm) and the 1H carboxylic acid proton (broad singlet, >10 ppm) will remain in the spectrum, although their exact chemical shifts might be slightly altered due to the change in the molecule’s electronic structure.
