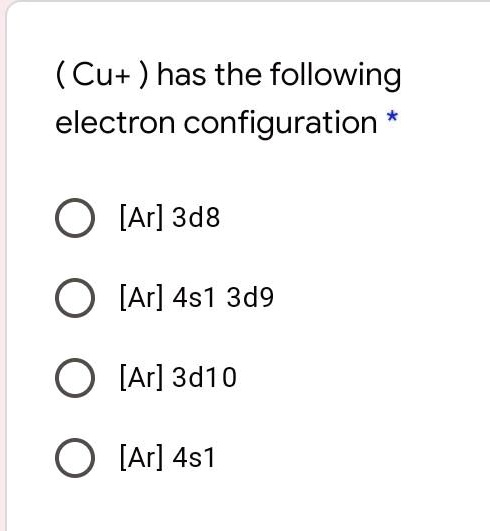
(Cu+) has the following electron configuration: [Ar] 3d^10 4s^1
The Correct Answer and Explanation is:
Correct Answer: [Ar] 3d¹⁰
Copper (Cu) has an atomic number of 29. In its neutral state, the expected electron configuration would follow the order of filling orbitals using the Aufbau principle. However, copper is an exception. Instead of filling the 4s orbital completely first, it arranges itself for stability. The neutral configuration becomes:
[Ar] 4s¹ 3d¹⁰
This arrangement provides a fully filled 3d subshell which is more stable than partially filled orbitals.
When copper loses one electron to form the Cu⁺ ion, the electron is removed from the 4s orbital first. The 4s orbital, although filled before the 3d orbital, is higher in energy once electrons occupy both subshells. Therefore, the electron from 4s¹ is removed, leaving:
[Ar] 3d¹⁰
This makes [Ar] 3d¹⁰ the correct electron configuration for Cu⁺.
The other options are incorrect for the following reasons:
- [Ar] 3d⁸ suggests two electrons were removed, which would be Cu²⁺, not Cu⁺.
- [Ar] 4s¹ 3d⁹ represents the electron arrangement before losing the 4s electron, so this is for neutral copper.
- [Ar] 4s¹ suggests only the 4s orbital is involved, ignoring the d electrons, which is inaccurate for copper.
Understanding copper’s electron behavior requires knowledge of exceptions in electron configurations. Transition metals often exhibit irregular configurations due to the stability offered by half-filled or fully filled d subshells. This unique behavior affects both the chemistry and the ion formation of elements like copper.
