Explain the completion of reaction based on the IR spectra of your product? (Attach IR spectra) 3-Nitro acetophenone (Starting Material) 3-Amino Acetophenone (PRODUCT)
The Correct Answer and Explanation is:
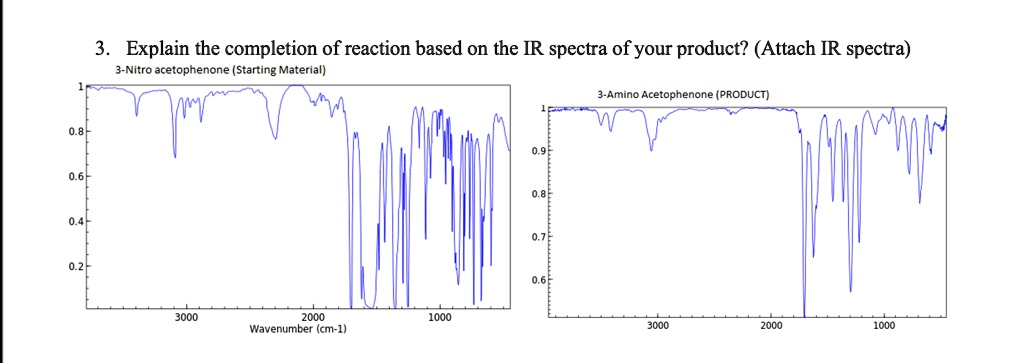
The IR spectra provide key insights into the completion of the reaction from 3-Nitro acetophenone to 3-Amino acetophenone by identifying changes in functional groups.
In the IR spectrum of 3-Nitro acetophenone, strong absorption bands appear around 1520 cm⁻¹ and 1350 cm⁻¹, which correspond to the asymmetric and symmetric stretching vibrations of the nitro group. Additionally, a peak near 1700 cm⁻¹ indicates the presence of the carbonyl functional group (C=O) characteristic of acetophenone. These peaks confirm the structure of the starting material.
Upon analyzing the IR spectrum of 3-Amino acetophenone, the disappearance of the nitro group peaks around 1520 cm⁻¹ and 1350 cm⁻¹ indicates that the nitro group has been successfully reduced or eliminated. Instead, a new broad absorption band appears around 3300 cm⁻¹, corresponding to the N-H stretching of the amino group. This peak confirms the presence of primary amine functionality, demonstrating that the nitro group has been converted into an amino group. Meanwhile, the carbonyl group absorption near 1700 cm⁻¹ remains unchanged, affirming that the acetophenone structure is still intact.
These spectral changes conclusively validate the completion of the reaction. The transition from nitro to amino functionality is evident, and the disappearance of the nitro-specific peaks combined with the appearance of the amino-specific peak confirms the conversion. The persistence of the carbonyl absorption further supports the integrity of the molecular backbone, ensuring that only the nitro group underwent transformation while the rest of the structure remained stable.
Thus, the IR spectra effectively demonstrate the successful conversion of 3-Nitro acetophenone to 3-Amino acetophenone, confirming the completion of the reaction through spectral evidence.
