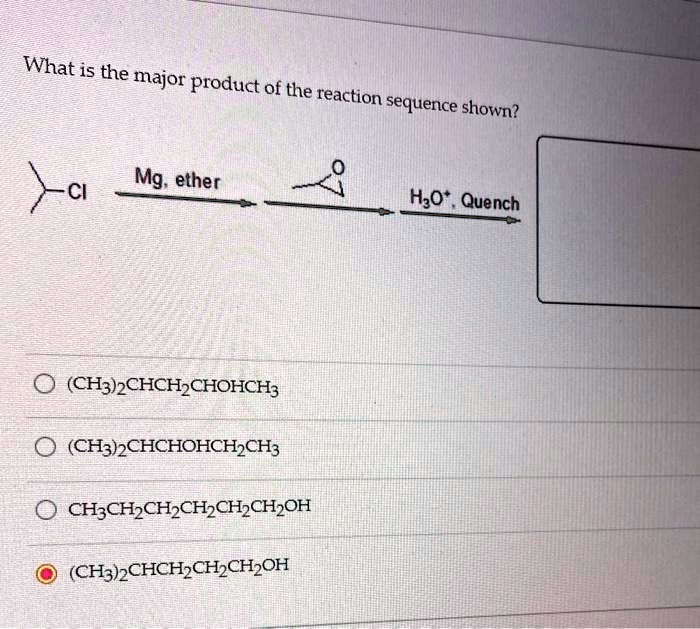
What is the major product of the reaction sequence shown? Mg, ether H2O. Quench (CH3)2CHCH2CHOHCH3 O (CH3)2CHCHOHCH3 CH3CH2CH2CH2CH2CH2OH (CH3)CHCH_CH-CH-OH
The Correct Answer and Explanation is:
The correct answer to the given reaction sequence is (CH3)2CHCH2CH2CH2OH.
Explanation:
The reaction begins with the formation of a Grignard reagent using magnesium metal in ether. The starting compound is (CH3)2CHCH2Cl, which reacts with magnesium to form (CH3)2CHCH2MgCl. This reaction introduces a nucleophilic carbon-metal bond, making the carbon highly reactive.
The next step involves the reaction of the Grignard reagent with an epoxide. Grignard reagents are strong nucleophiles, meaning they attack electrophilic centers readily. In this case, the reagent targets the less substituted carbon of the epoxide, leading to ring opening. This step extends the carbon chain by introducing an additional oxygen-bound carbon.
Once the epoxide is opened, the intermediate formed is an alkoxide. The final step involves quenching this alkoxide with H3O+, which provides protons to neutralize the negatively charged oxygen. This step results in the formation of an alcohol at the site of epoxide opening.
The major product is (CH3)2CHCH2CH2CH2OH, which is a primary alcohol. This result follows the general principle that Grignard reagents react with epoxides at the less hindered site, leading to an extension of the carbon chain by two additional carbons and the incorporation of a hydroxyl group.
This reaction is widely used in organic synthesis to extend carbon skeletons and introduce functional groups essential for further chemical transformations. It demonstrates the utility of Grignard reagents in forming alcohols efficiently while controlling regioselectivity in ring-opening reactions.
