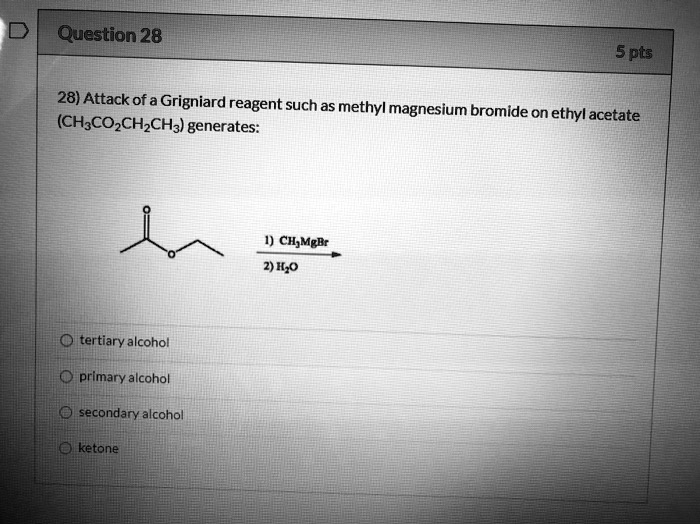
Attack of a Grignard reagent such as methylmagnesium bromide (CH3CO2CH2CH3) generates on ethyl acetate: CH3MgBr 2) H2O tertiary alcohol primary alcohol secondary alcohol ketone
The Correct Answer and Explanation is:
The correct answer is tertiary alcohol.
Explanation:
The reaction shown is the addition of a Grignard reagent, methylmagnesium bromide (CH₃MgBr), to an ester, ethyl acetate (CH₃COOCH₂CH₃). This reaction proceeds in two main stages, ultimately resulting in the formation of a tertiary alcohol.
- First Nucleophilic Attack and Ketone Formation: The methyl group (CH₃) in the Grignard reagent acts as a potent nucleophile. It attacks the electrophilic carbonyl carbon of the ethyl acetate. This initial attack breaks the carbon-oxygen double bond and forms a tetrahedral intermediate. This intermediate is unstable and quickly collapses. The ethoxy group (⁻OCH₂CH₃) is a good leaving group, so it is expelled, and the carbon-oxygen double bond reforms. The product of this first step is a ketone, in this case, acetone (CH₃COCH₃).
- Second Nucleophilic Attack and Alcohol Formation: The reaction does not stop at the ketone. Ketones are also highly reactive towards Grignard reagents. A second molecule of methylmagnesium bromide attacks the carbonyl carbon of the newly formed acetone. This second nucleophilic attack forms a new tetrahedral intermediate, which is a magnesium alkoxide salt.
- Protonation (Aqueous Workup): The final step is the addition of water (H₂O). The negatively charged oxygen of the alkoxide salt is a strong base and is protonated by water. This step neutralizes the intermediate and generates the final alcohol product. In this specific reaction, the product is 2-methyl-2-propanol, also known as tert-butyl alcohol.
The carbon atom bonded to the hydroxyl (-OH) group in tert-butyl alcohol is also bonded to three other carbon atoms (three methyl groups). By definition, an alcohol in which the hydroxyl-bearing carbon is attached to three other carbons is a tertiary alcohol
