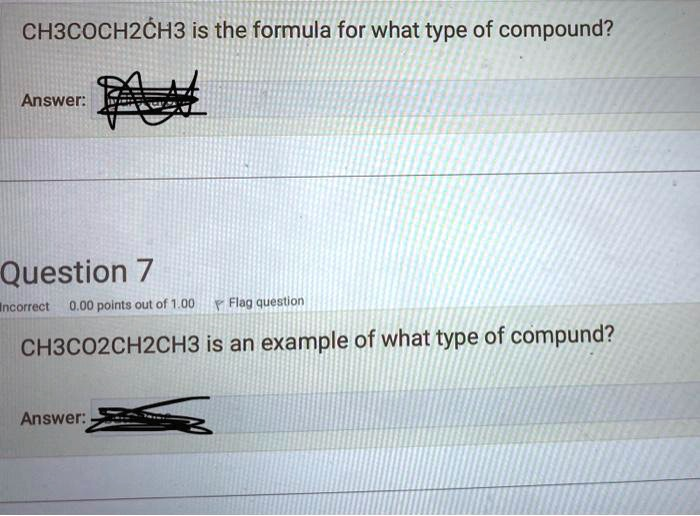
CH3COCH2CH3 is the formula for what type of compound? Answer: Question 7 Incorrect 0.00 points out of 1.00 Flag question CH3CO2CH2CH3 is an example of what type of compund? Answer:
The Correct Answer and Explanation is:
Based on the chemical formulas provided in the image, here are the correct answers:
First Question: CH3COCH2CH3 is the formula for a ketone.
Question 7: CH3CO2CH2CH3 is an example of an ester.
Explanation
The classification of these organic compounds depends on identifying their specific functional groups, which are the reactive centers of the molecules.
The first formula, CH3COCH2CH3, represents a ketone. The key functional group in this molecule is the carbonyl group, represented by ‘CO’. A carbonyl group consists of a carbon atom double-bonded to an oxygen atom (C=O). In a ketone, this carbonyl group is bonded to two other carbon atoms. For the molecule CH3COCH2CH3, the carbonyl carbon is attached to a methyl group (CH3) on one side and an ethyl group (CH2CH3) on the other. The general formula for a ketone is R-CO-R’, where R and R’ are alkyl or aryl groups. Because the carbonyl group is positioned internally within the carbon chain, this compound is classified as a ketone. Its specific name is butan-2-one or ethyl methyl ketone.
The second formula, CH3CO2CH2CH3, represents an ester. The question was marked incorrect on the quiz, which means the student’s answer was not “ester.” The key to identifying this compound is the ‘CO2′ part, which is better understood structurally as a carbonyl group (C=O) where the carbonyl carbon is also single-bonded to a second oxygen atom. This entire -COO- arrangement is the ester functional group. The general formula for an ester is R-COO-R’. In this molecule, the R group is a methyl group (CH3) and the R’ group is an ethyl group (CH2CH3). Esters are typically formed from the reaction between a carboxylic acid and an alcohol. This specific compound, ethyl ethanoate (also known as ethyl acetate), is derived from ethanoic acid and ethanol. The crucial difference between a ketone and an ester is that an ester has an oxygen atom directly attached to the carbonyl carbon, while a ketone does not. This structural difference gives esters distinct chemical properties and they are often known for their pleasant, fruity smells
