‘Identify the parts of the atom that are labeled in the diagram
The Correct Answer and Explanation is:
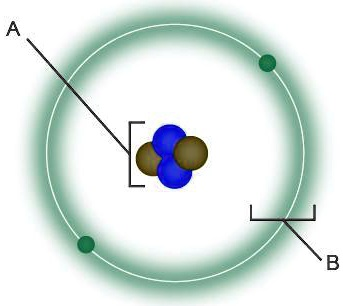
Based on the provided diagram of an atom, the labeled parts are:
- A: Electron
- B: Electron Shell
Explanation
This diagram illustrates a simplified model of an atom, showing its fundamental components.
A points to an electron. The electron is a subatomic particle that carries a negative electrical charge. Electrons are extremely small and have very little mass compared to the particles in the center of the atom. They exist in the space surrounding the atom’s core in specific regions of probability. In this Bohr model representation, electrons are shown moving in a distinct circular path around the center. The number and arrangement of an atom’s electrons, particularly those in its outermost layer, determine the atom’s chemical behavior and how it will bond with other atoms.
B indicates the electron shell. An electron shell, also called an energy level or orbital, is the region or path where electrons are found as they move around the atom’s center. Each shell is associated with a specific energy level, and shells farther from the center have higher energy. There is a maximum number of electrons that each shell can accommodate; the first shell, shown here, can hold up to two electrons. The visual representation of the shell as a thin line indicates a fixed orbit, which is characteristic of the Bohr model. However, the green, hazy glow surrounding the path suggests the more modern quantum concept of an “electron cloud,” a three dimensional space where the electrons are most likely to be located at any given moment.
The unlabeled central part, bracketed and containing blue and brown spheres, is the nucleus. This is the dense, positively charged core of the atom. It is composed of protons (positively charged particles, likely the blue spheres) and neutrons (neutral particles with no charge, likely the brown spheres). The nucleus contains almost all of the atom’s mass
