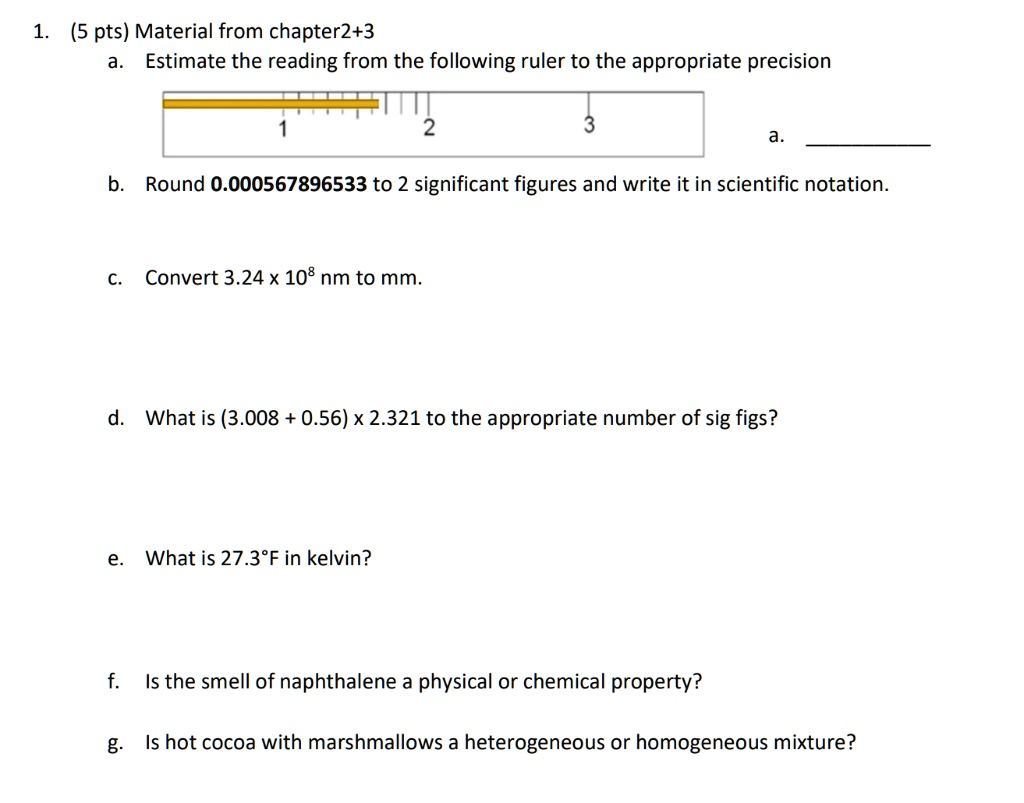
Material from Chapter 2+3: Estimate the reading from the following ruler to the appropriate precision. Round 0.000567896533 to 2 significant figures and write it in scientific notation. Convert 3.24 x 10^8 nm to mm. What is (3.008 + 0.56) x 2.321 to the appropriate number of sig figs? What is 27.3°F in Kelvin? Is the smell of naphthalene a physical or chemical property? Is hot cocoa with marshmallows a heterogeneous or homogeneous mixture?
The Correct Answer and Explanation is:
Here are the correct answers and a detailed explanation for each question.
a. 1.85
To determine the reading with the correct precision, we first identify the smallest markings on the ruler. The space between 1 and 2 is divided into 10 increments, meaning each mark represents 0.1 units. The measurement must be recorded to one decimal place beyond the smallest marking. We can say with certainty that the orange bar is past the 1.8 mark but has not reached the 1.9 mark. Therefore, the first two digits are certain: 1.8. The final digit must be estimated. The end of the bar appears to be exactly halfway between the 1.8 and 1.9 marks, so the best estimation for the hundredths place is 5. This gives a final reading of 1.85.
b. 5.7 x 10⁻⁴
To round 0.000567896533 to two significant figures, we identify the first two non-zero digits, which are 5 and 6. We then look at the next digit, 7. Since 7 is 5 or greater, we round the preceding digit (6) up to 7. The rounded number is 0.00057. To express this in scientific notation, we move the decimal point four places to the right to get a number between 1 and 10, which is 5.7. Because we moved the decimal to the right, the exponent is negative, resulting in 5.7 x 10⁻⁴.
c. 3.24 x 10² mm
To convert nanometers (nm) to millimeters (mm), we can use meters (m) as an intermediate unit. There are 10⁹ nm in 1 m, and 10³ mm in 1 m.
(3.24 x 10⁸ nm) × (1 m / 10⁹ nm) × (10³ mm / 1 m) = 3.24 x 10⁽⁸ ⁻ ⁹ ⁺ ³⁾ mm = 3.24 x 10² mm.
d. 8.29
Following the order of operations, we first perform the addition inside the parentheses: 3.008 + 0.56 = 3.568. According to the rules for addition with significant figures, the result must be rounded to the same number of decimal places as the number with the fewest decimal places (0.56 has two). So, 3.568 is rounded to 3.57. Next, we multiply 3.57 by 2.321. For multiplication, the answer must have the same number of significant figures as the factor with the fewest significant figures. 3.57 has three, and 2.321 has four. Therefore, the final answer must have three significant figures. 3.57 × 2.321 = 8.28607, which rounds to 8.29.
e. 270.5 K
To convert Fahrenheit to Kelvin, you must first convert to Celsius. The formula is °C = (°F – 32) / 1.8.
°C = (27.3 – 32) / 1.8 = -4.7 / 1.8 = -2.611… °C.
Next, convert Celsius to Kelvin using the formula K = °C + 273.15.
K = -2.611… + 273.15 = 270.538… K.
For precision, the subtraction step (27.3 – 32 = -4.7) results in a number precise to the tenths place. Thus, the final answer should also be rounded to the tenths place, giving 270.5 K.
f. Physical property
The smell of a substance is a physical property. A physical property can be observed or measured without changing the chemical composition of the substance. When you smell naphthalene, gaseous naphthalene molecules travel to your nose, but they are still naphthalene molecules. No chemical reaction occurs.
g. Heterogeneous mixture
Hot cocoa with marshmallows is a heterogeneous mixture. In a heterogeneous mixture, the different components are visibly distinguishable and not uniformly distributed. You can clearly see the solid marshmallows as a separate phase from the liquid hot cocoa. If it were a homogeneous mixture, the components would be uniformly mixed down to the molecular level, and you would not be able to see the individual parts
