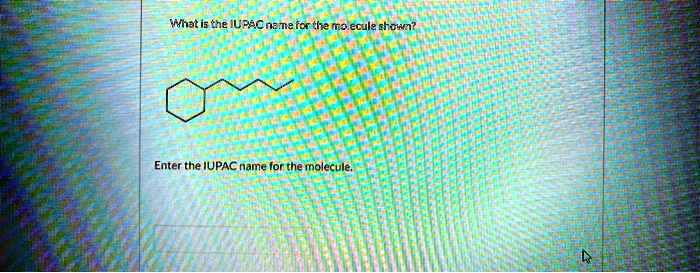
What is the IUPAC name for the molecule shown?
The Correct Answer and Explanation is:
butylcyclohexane
To determine the International Union of Pure and Applied Chemistry (IUPAC) name for the molecule shown, we must follow a systematic process.
- Identify the main structural features. The molecule is composed of a six-membered ring and an attached unbranched alkyl chain. This identifies the molecule as a substituted cycloalkane.
- Determine the parent structure. For a cycloalkane with a single alkyl substituent, the parent structure is determined by comparing the number of carbon atoms in the ring to the number of carbon atoms in the chain. The component with the higher number of carbon atoms becomes the parent name.
- The ring is a hexagon, which signifies a six-carbon cyclic structure. Since there are no double or triple bonds, its name is cyclohexane. It contains 6 carbon atoms.
- The attached alkyl chain has four carbon atoms (counting each vertex and the endpoint of the line-angle drawing). A four-carbon alkyl group is called a butyl group. It contains 4 carbon atoms.
- Compare the carbon counts. The cyclohexane ring (6 carbons) has more carbon atoms than the butyl chain (4 carbons). Therefore, the cyclohexane ring is the parent structure, and the butyl chain is considered a substituent.
- Assemble the final name. The name of the substituent is placed as a prefix before the name of the parent structure. Thus, we combine “butyl” and “cyclohexane” to get butylcyclohexane.
In the case of a monosubstituted cycloalkane, a locant number (like “1-“) is not required. This is because all positions on the ring are chemically equivalent, and the single substituent is, by definition, located at position 1. Therefore, “1-butylcyclohexane” is a valid name, but the simpler name “butylcyclohexane” is preferred by IUPAC.
