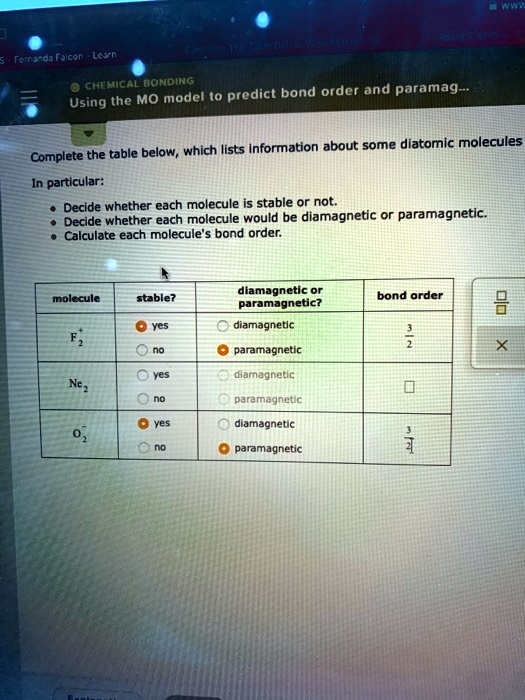
Chemical Bonding: Predicting Bond Order and Paramagnetism Using the MO Model To determine the stability and magnetic properties of diatomic molecules, we can use the molecular orbital (MO) model. The table below provides information about some diatomic molecules. Please complete the table by deciding whether each molecule is stable or not, whether it would be diamagnetic or paramagnetic, and calculating the bond order for each molecule. Molecule Stable? Bond Order Magnetic Properties ——————————————————– Yes Diamagnetic No Paramagnetic Yes Diamagnetic Yes Paramagnetic No Diamagnetic Yes Paramagnetic
The Correct Answer and Explanation is:
Based on the principles of Molecular Orbital (MO) theory, here are the completed answers for the table and a detailed explanation.
Correct Answer
Here is the completed table with the correct properties for each diatomic molecule:
| Molecule | Stable? | Diamagnetic or Paramagnetic? | Bond Order |
| F₂⁺ | yes | paramagnetic | 1.5 |
| Ne₂ | no | diamagnetic | 0 |
| O₂⁻ | yes | paramagnetic | 1.5 |
Explanation
To determine the properties of these diatomic molecules, we use the Molecular Orbital (MO) model. The stability of a molecule is determined by its bond order; if the bond order is greater than zero, the molecule is considered stable. The magnetic property depends on the presence of unpaired electrons; if there are unpaired electrons, the molecule is paramagnetic, and if all electrons are paired, it is diamagnetic. The bond order is calculated as:
Bond Order = ½ (Number of bonding electrons – Number of anti-bonding electrons)
The order of filling for the valence molecular orbitals for O₂, F₂, and Ne₂ is:
σ(2s), σ*(2s), σ(2p), π(2p), π*(2p), σ*(2p)
1. F₂⁺ (Fluoride Cation)
- Valence Electrons: A neutral fluorine atom (F) has 7 valence electrons. F₂ has 14, and the F₂⁺ ion has lost one electron, giving it a total of 13 valence electrons.
- MO Configuration: (σ2s)² (σ2s)² (σ2p)² (π2p)⁴ (π2p)³
- Bond Order: There are 8 bonding electrons (in σ2s, σ2p, π2p) and 5 anti-bonding electrons (in σ2s, π2p).
Bond Order = ½ (8 – 5) = 1.5. Since the bond order is > 0, F₂⁺ is stable. - Magnetic Properties: The (π*2p)³ orbital contains one unpaired electron. Therefore, F₂⁺ is paramagnetic.
2. Ne₂ (Dineon)
- Valence Electrons: A neutral neon atom (Ne) has 8 valence electrons. The Ne₂ molecule has 8 + 8 = 16 valence electrons.
- MO Configuration: (σ2s)² (σ2s)² (σ2p)² (π2p)⁴ (π2p)⁴ (σ*2p)²
- Bond Order: There are 8 bonding electrons and 8 anti-bonding electrons.
Bond Order = ½ (8 – 8) = 0. A bond order of zero indicates that no net bond is formed, so Ne₂ is unstable. - Magnetic Properties: All molecular orbitals are completely filled, meaning there are no unpaired electrons. Therefore, Ne₂ is diamagnetic.
3. O₂⁻ (Superoxide Ion)
- Valence Electrons: A neutral oxygen atom (O) has 6 valence electrons. O₂ has 12, and the O₂⁻ ion has gained one electron, giving it a total of 13 valence electrons.
- MO Configuration: (σ2s)² (σ2s)² (σ2p)² (π2p)⁴ (π2p)³
- Bond Order: There are 8 bonding electrons and 5 anti-bonding electrons.
Bond Order = ½ (8 – 5) = 1.5. Since the bond order is > 0, O₂⁻ is stable. - Magnetic Properties: The (π*2p)³ orbital has one unpaired electron, making O₂⁻ paramagnetic.
