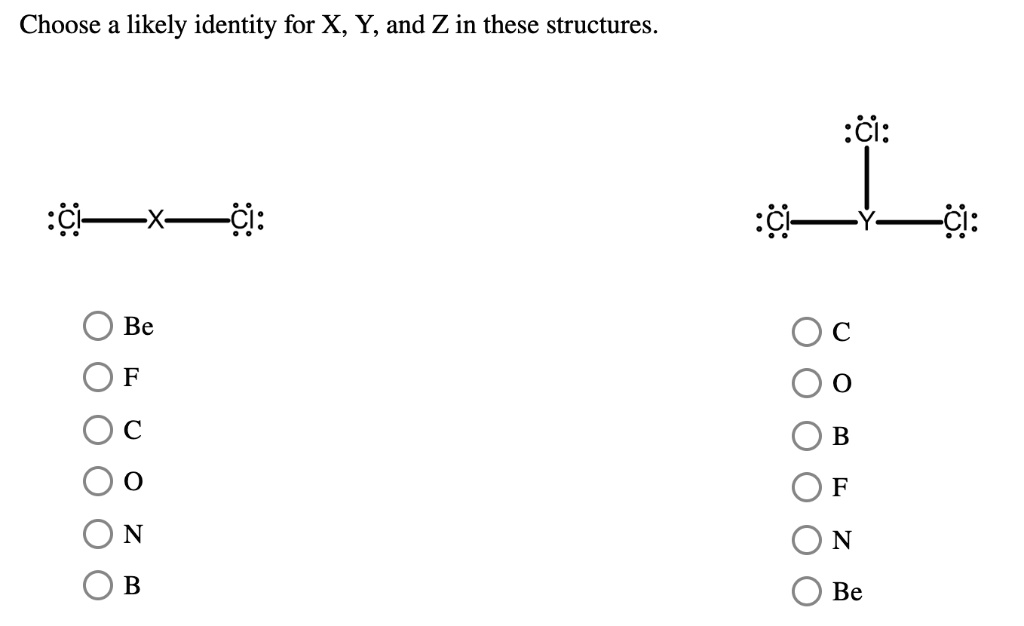
Choose a likely identity for X, Y, and Z in these structures. :Cl:: -X- :Cl:: Be F C O N B :Cl:: -Y- :Cl:: C O B F N Be
The Correct Answer and Explanation is:
Correct Answer:
For the structure on the left (Cl-X-Cl), the correct identity for X is Be (Beryllium).
For the structure on the right (with central atom Y), the correct identity for Y is B (Boron).
Explanation:
To determine the likely identity of atoms X and Y, we must analyze their bonding characteristics as depicted in the provided Lewis structures. This involves counting the number of bonds they form and the number of lone pair electrons they possess, which in turn reveals the number of valence electrons each atom contributes.
Identifying Atom X:
In the first structure, :Cl-X-Cl:, the central atom X forms two single covalent bonds, one with each chlorine atom. The Lewis structure shows no lone pairs of electrons on X. In a covalent bond, each atom typically contributes one electron. Since X forms two bonds, it must contribute two of its valence electrons.
Let’s evaluate the options for X based on their valence electrons:
- Be (Beryllium): Group 2, 2 valence electrons.
- B (Boron): Group 13, 3 valence electrons.
- C (Carbon): Group 14, 4 valence electrons.
- N (Nitrogen): Group 15, 5 valence electrons.
- O (Oxygen): Group 16, 6 valence electrons.
- F (Fluorine): Group 17, 7 valence electrons.
Beryllium (Be) is the only element on the list with two valence electrons, making it the perfect fit for atom X. The resulting molecule, BeCl₂, is a known stable compound. Although beryllium does not satisfy the octet rule (it only has four electrons in its valence shell), it is a common exception.
Identifying Atom Y:
In the second structure, the central atom Y is bonded to three chlorine atoms via single bonds. Again, there are no lone pairs shown on the central atom Y. This means Y contributes one valence electron to each of the three bonds, for a total of three valence electrons.
Reviewing the list of options for Y, we find that Boron (B), being in Group 13, has exactly three valence electrons. This makes it the most plausible identity for Y. The molecule shown would be boron trichloride (BCl₃). Like beryllium, boron is another well-known exception to the octet rule, being stable with six electrons in its valence shell.
The question also mentions identifying “Z”, but no third structure is provided, indicating this is likely a typographical error in the problem statement. Based on the given diagrams, the correct choices are Be for X and B for Y.Correct Answer:**
For the structure containing X, the correct answer is Be.
For the structure containing Y, the correct answer is B.
Explanation
To determine the identity of the central atoms X and Y, we must analyze the number of valence electrons each atom contributes to the bonds shown in the Lewis structures. We will assume the molecules are neutral, meaning the formal charge on the central atom should ideally be zero. The formal charge of an atom in a molecule is calculated using the formula:
Formal Charge = (Number of Valence Electrons) – (Number of Non-bonding Electrons) – (1/2 * Number of Bonding Electrons)
Analysis of Element X:
The first Lewis structure shows a central atom, X, forming a single covalent bond with two chlorine atoms. There are no lone pairs of electrons depicted on atom X.
- Bonds and Electrons: X has 2 single bonds, meaning there are 4 bonding electrons around it. It has 0 non-bonding electrons (lone pair electrons).
- Formal Charge Calculation: To find the required number of valence electrons (V) for X to have a formal charge of zero, we apply the formula:
Formal Charge(X) = V – 0 – (1/2 * 4)
0 = V – 2
V = 2 - Identifying the Element: We need to find the element from the given options that has 2 valence electrons.
- Be (Beryllium): Group 2, 2 valence electrons.
- F, C, O, N, B have 7, 4, 6, 5, and 3 valence electrons, respectively.
- Conclusion: Beryllium (Be) is the only element that fits this requirement. The molecule BeCl₂ is a known stable compound, although Be is an exception to the octet rule, having only four electrons in its valence shell.
Analysis of Element Y:
The second Lewis structure shows a central atom, Y, forming single covalent bonds with three chlorine atoms. Similar to X, there are no lone pairs of electrons on atom Y.
- Bonds and Electrons: Y has 3 single bonds, which accounts for 6 bonding electrons. It has 0 non-bonding electrons.
- Formal Charge Calculation: We calculate the required number of valence electrons (V) for Y to have a formal charge of zero:
Formal Charge(Y) = V – 0 – (1/2 * 6)
0 = V – 3
V = 3 - Identifying the Element: We now look for the element among the options for Y that has 3 valence electrons.
- B (Boron): Group 13, 3 valence electrons.
- C, O, F, N, Be have 4, 6, 7, 5, and 2 valence electrons, respectively.
- Conclusion: Boron (B) is the correct identity for Y. The molecule BCl₃ is a well-known compound where Boron, another exception to the octet rule, is stable with six valence electrons.
The question asks for the identity of Z, but no structure containing Z is provided. This is most likely a typographical error in the problem statement. Based on the two structures given, X is Beryllium (Be) and Y is Boron (B). a neutral central atom can be determined by the bonds it forms and its lone pairs. In this case, X contributes one electron to each of the two single bonds. Since there are no lone pairs, X must have 2 valence electrons.
4. Analyzing the Options:
* Be (Beryllium): A Group 2 element with 2 valence electrons. It typically forms two single bonds, resulting in a molecule like BeCl₂. This structure is an exception to the octet rule, as Be only has four electrons in its valence shell, which matches the diagram perfectly.
* Other options like Carbon (4 valence e⁻), Oxygen (6 valence e⁻), or Boron (3 valence e⁻) do not fit this bonding pattern. For instance, Oxygen would have two lone pairs in addition to two bonds (as in OCl₂).
Therefore, X is most likely Beryllium (Be).
Identifying Atom Y:
The second structure shows a central atom, Y, forming a single covalent bond with three chlorine (Cl) atoms in a trigonal planar arrangement.
- Bonding: Atom Y forms three single bonds. To do this, it must contribute at least three valence electrons.
- Lone Pairs: The structure shows no lone pairs of electrons on atom Y.
- Valence Electrons: Atom Y contributes one electron to each of the three single bonds. With no lone pairs, Y must have 3 valence electrons.
- Analyzing the Options:
- B (Boron): A Group 13 element with 3 valence electrons. It commonly forms three single bonds, creating molecules like BCl₃. This is another common exception to the octet rule, where Boron has only six electrons in its valence shell. This fits the provided diagram exactly.
- Other options do not fit. Nitrogen (5 valence e⁻) would form three bonds but would also have one lone pair (as in NCl₃) to satisfy the octet rule. Carbon (4 valence e⁻) typically forms four bonds.
Therefore, Y is most likely Boron (B).
(Note: The question mentions identifying “Z”, but no structure containing Z is provided. The analysis is based on the two structures shown.
