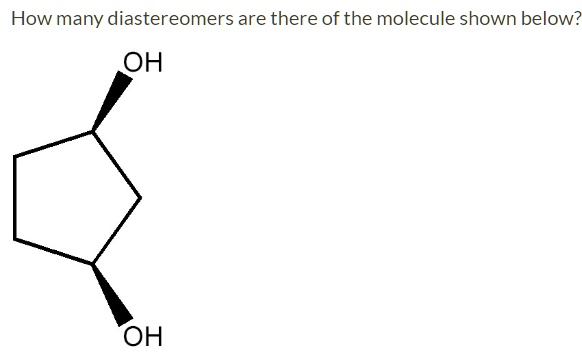
How many diastereomers are there of the molecule shown below? OH
The Correct Answer and Explanation is:
To determine the number of diastereomers of the given molecule, we first need to analyze its structure and identify all its possible stereoisomers.
- Identify the Stereocenters: The molecule is cyclopentane-1,3-diol. The carbon atoms at positions 1 and 3, which are bonded to the hydroxyl (-OH) groups, are both chiral centers (or stereocenters). Each is attached to four different groups: an -OH group, a hydrogen atom (implied), and two different carbon pathways within the ring. With two stereocenters (n=2), the maximum possible number of stereoisomers is 2^n = 2^2 = 4.
- Analyze the Given Isomer: The image shows a specific stereoisomer where both -OH groups are pointing towards the viewer (indicated by the solid wedges). This is the cis isomer. We must check for internal symmetry. This cis-1,3-cyclopentanediol molecule has a plane of symmetry that passes through the C2 carbon and bisects the C4-C5 bond. A molecule with chiral centers and a plane of symmetry is called a meso compound. Meso compounds are achiral and are superimposable on their mirror images. Therefore, there is only one cis isomer.
- Identify Other Stereoisomers: The other possible stereoisomers are the trans isomers, where one -OH group points up (wedge) and the other points down (dash). The trans configuration lacks a plane of symmetry, making it chiral. A chiral molecule is non-superimposable on its mirror image. Consequently, the trans isomer exists as a pair of enantiomers (non-superimposable mirror images).
- Count Total Stereoisomers: In total, there are three stereoisomers of cyclopentane-1,3-diol:
- The single cis isomer (a meso compound), which is the molecule shown in the question.
- A pair of trans enantiomers.
- Determine the Number of Diastereomers: The question asks for the number of diastereomers of the given molecule (the cis-meso isomer). Diastereomers are stereoisomers that are not mirror images of each other.
- The relationship between the cis (meso) isomer and either of the trans enantiomers is that they are stereoisomers but not mirror images.
- Therefore, both trans enantiomers are diastereomers of the original cis molecule.
The given molecule has two diastereomers: the two chiral trans isomers.
