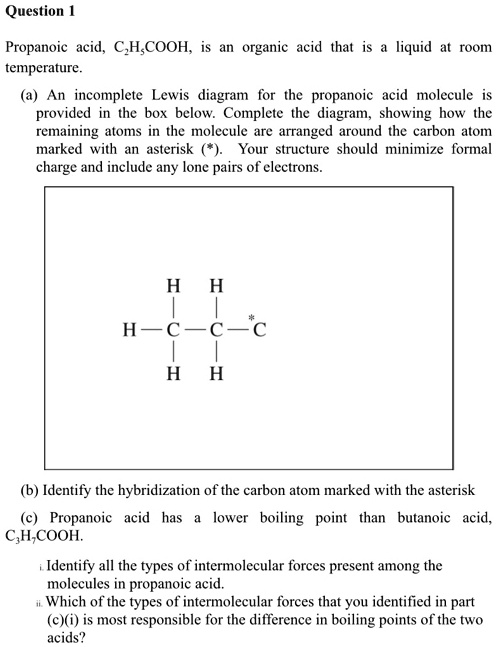
Propanoic acid, C3H6COOH, is an organic acid that is liquid at room temperature. An incomplete Lewis diagram for the propanoic acid molecule is provided in the box below. Complete the diagram, showing how the remaining atoms in the molecule are arranged around the carbon atom marked with an asterisk. Your structure should minimize formal charge and include any lone pairs of electrons. H | C-*-C | H (b) Identify the hybridization of the carbon atom marked with the asterisk. (c) Propanoic acid has a lower boiling point than butanoic acid, CH3CH2COOH. Identify all the types of intermolecular forces present among the molecules in propanoic acid. Which of the types of intermolecular forces that you identified in part (c)(i) is most responsible for the difference in boiling points of the two acids?
The Correct Answer and Explanation is:
Here are the correct answers for each part of the question.
(a) Completed Lewis Diagram Description:
The carbon atom marked with an asterisk is bonded to three other atoms. It forms a double bond with one oxygen atom and a single bond with a second oxygen atom. This second oxygen atom is also bonded to a hydrogen atom. The oxygen in the double bond has two lone pairs of electrons, and the oxygen in the single bond also has two lone pairs.
(b) Hybridization:
sp2
(c) Intermolecular Forces and Boiling Points:
i. The types of intermolecular forces present in propanoic acid are London dispersion forces, dipole-dipole interactions, and hydrogen bonding.
ii. London dispersion forces are most responsible for the difference
ii. Which of the types of intermolecular forces that you identified in part (c)(i) is most responsible for the difference in boiling points of the two acids?
The intermolecular force most responsible for the difference in boiling points between propanoic acid and butanoic acid is London dispersion forces (LDF).
Propanoic acid (CH₃CH₂COOH) and butanoic acid (CH₃CH₂CH₂COOH) are both carboxylic acids. They share the same functional group, the carboxyl group (-COOH). This means both molecules can participate in strong hydrogen bonding and dipole-dipole interactions of very similar strength. Hydrogen bonding, in particular, is the strongest type of intermolecular force present in both substances, and it is the primary reason for their relatively high boiling points compared to alkanes of similar molar mass.
However, the question asks what accounts for the difference in their boiling points. The structural difference between the two molecules is that butanoic acid has one additional methylene group (-CH₂-) in its hydrocarbon chain. This makes butanoic acid a larger molecule with a greater surface area and more electrons than propanoic acid. The strength of London dispersion forces increases with the size of the electron cloud and the surface area of the molecule. Therefore, butanoic acid experiences stronger London dispersion forces than propanoic acid. Because the overall intermolecular forces (the sum of hydrogen bonding, dipole-dipole, and LDFs) are stronger in butanoic acid, more energy is required to separate its molecules and transition from the liquid to the gas phase, resulting in a higher boiling point. in boiling points between propanoic acid and butanoic acid.
Explanation
(a) Completing the Lewis Diagram
The question asks to complete the structure for propanoic acid (C2H5COOH) around the asterisked carbon. The incomplete diagram shows the ethyl group (C2H5) attached to this carbon. This carbon is part of the carboxyl functional group (-COOH). To complete the structure while satisfying the octet rule and minimizing formal charge, the asterisked carbon must form a double bond with one oxygen atom and a single bond with the other oxygen atom. The second oxygen atom is then bonded to the final hydrogen atom. To complete their octets, the oxygen atom in the C=O double bond requires two lone pairs of electrons, and the oxygen atom in the C-O-H group also requires two lone pairs.
(b) Hybridization of the Asterisked Carbon
The hybridization of an atom is determined by the number of sigma bonds and lone pairs around it. The asterisked carbon atom forms three sigma bonds (one C-C single bond, one C-O single bond, and one of the bonds in the C=O double bond) and has no lone pairs of electrons. The total of three regions of electron density corresponds to sp2 hybridization. This hybridization results in a trigonal planar geometry around the carbon atom, with bond angles of approximately 120 degrees.
(c) Intermolecular Forces and Boiling Point Difference
i. Propanoic acid molecules are held together by three types of intermolecular forces. First, as with all molecules, there are London dispersion forces (LDF), which arise from temporary fluctuations in electron density. Second, because propanoic acid contains polar C=O and
