Starting with the ester shown below, synthesize a ?-ketoester, hydrolyze ester back to the carboxylic acid, and perform the decarboxylation. Ketone 1) CH
CH
O
2) H
Heat ?-ketoester H
H
O ?-ketocarboxylic acid
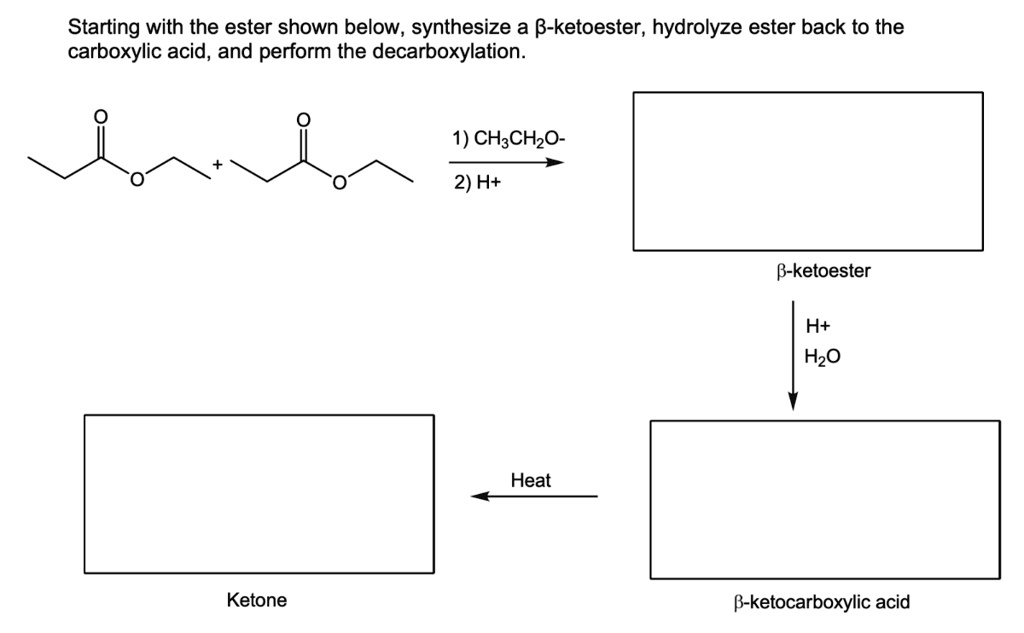
The Correct Answer and Explanation is:
Here are the completed chemical structures for the reaction sequence.
Explanation
The reaction sequence outlines a method for synthesizing a ketone from an ester, involving three main steps: a Claisen condensation, ester hydrolysis, and decarboxylation.
1. Synthesis of the β-ketoester: The first step is a Claisen condensation. Two molecules of the starting material, ethyl propanoate, react in the presence of a strong base, sodium ethoxide (CH3CH2O-). The ethoxide removes an acidic α-proton from one ester molecule to form a nucleophilic enolate. This enolate then attacks the electrophilic carbonyl carbon of a second ester molecule. The resulting tetrahedral intermediate collapses, eliminating an ethoxide ion to form the β-ketoester product, ethyl 2-methyl-3-oxopentanoate. An acidic workup (H+) is required in the second part of this step to neutralize the reaction mixture and protonate the enolate of the product, yielding the final neutral β-ketoester.
2. Hydrolysis to a β-ketocarboxylic acid: The β-ketoester is then treated with acid (H+) and water. This is an acid-catalyzed ester hydrolysis reaction. The ethyl ester group (–COOCH2CH3) is converted into a carboxylic acid group (–COOH). This transformation yields 2-methyl-3-oxopentanoic acid, which is a β-ketocarboxylic acid because the ketone group is on the beta-carbon relative to the carboxyl group.
3. Decarboxylation to a Ketone: The final step involves heating the β-ketocarboxylic acid. Molecules of this type are thermally unstable and readily undergo decarboxylation, which is the loss of a molecule of carbon dioxide (CO2). The reaction proceeds through a stable, cyclic six-membered transition state. This process results in the formation of an enol intermediate, which rapidly rearranges (tautomerizes) to the more stable final product, the ketone 3-pentanone. This overall sequence is a variation of the acetoacetic ester synthesis, a powerful method for preparing substituted ketones.
