Which is the major product of the following reaction
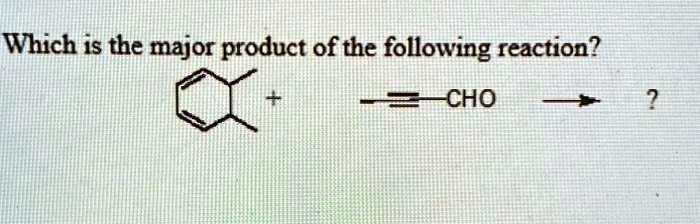
The Correct Answer and Explanation is:
Of course. Here is the correct answer and a detailed explanation.
The major product is the endo isomer of 1,2-dimethyl-3-formylbicyclo[2.2.2]octa-2,5-diene.
Explanation:
This reaction is a classic example of a Diels-Alder reaction, which is a type of [4+2] cycloaddition. In this reaction, a conjugated diene (a molecule with two alternating double bonds) reacts with a dienophile (a molecule with a double or triple bond) to form a six-membered ring.
- Reactants Analysis:
- The Diene: The molecule on the left is 1,2-dimethyl-1,3-cyclohexadiene. The two double bonds are part of a six-membered ring, which locks them in the s-cis conformation. This specific shape is required for the Diels-Alder reaction to occur. The two methyl groups (-CH3) are electron-donating groups, which make the diene electron-rich and more reactive.
- The Dienophile: The molecule on the right is propynal. It contains a triple bond (an alkyne) which will act as the two-electron component (the “phile”). The aldehyde group (-CHO) is a strong electron-withdrawing group. This makes the dienophile electron-poor and highly reactive towards an electron-rich diene. The reaction between an electron-rich diene and an electron-poor dienophile is highly favored.
- Reaction Mechanism and Product Formation:
The reaction is concerted, meaning all bonds are formed and broken in a single step. The four pi electrons from the diene and two pi electrons from the alkyne dienophile reorganize to form a new, more stable bicyclic structure.- Two new single bonds (sigma bonds) form, creating a new six-membered ring fused to the original ring.
- Since the dienophile is an alkyne (triple bond), the resulting new ring in the product will contain a double bond. This leads to the formation of a bicyclo[2.2.2]octadiene skeleton.
- Stereochemistry and the Endo Rule:
The most important factor in determining the major product is the stereochemistry. The Diels-Alder reaction is stereospecific and preferentially forms the endo product.- The Endo Rule states that the electron-withdrawing substituent on the dienophile (the -CHO group in this case) will orient itself “under” the diene in the transition state. This orientation places it on the same side as the larger bridge of the bicyclic system.
- This preference is not due to sterics (the exo product is actually less sterically hindered), but to a stabilizing electronic effect called secondary orbital overlap. The p-orbitals of the aldehyde’s carbonyl group can interact favorably with the p-orbitals of the developing double bond in the diene during the transition state. This lowers the activation energy for the endo pathway, making it the kinetically favored and therefore the major product under normal conditions.
