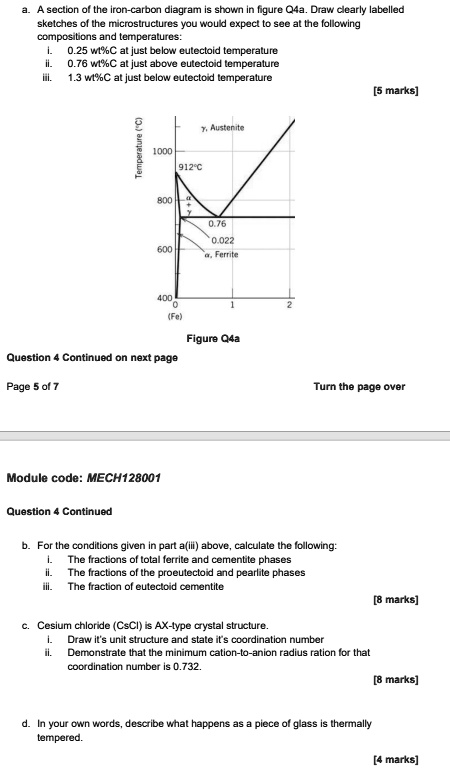
A section of the iron-carbon diagram is shown in figure Q4a. Draw clearly labelled sketches of the microstructures you would expect to see at the following compositions and temperatures: i. 0.25 wt%C at just below eutectoid temperature ii. 0.76 wt%C at just above eutectoid temperature iii. 1.3 wt%C at just below eutectoid temperature [5 marks] Question 4 Continued on next page Page 5 of 7 Turn the page over Module code: MECH128001 Question 4 Continued b. For the conditions given in part a(iii) above, calculate the following: i. The fractions of total ferrite and cementite phases ii. The fractions of the proeutectoid and pearlite phases iii. The fraction of eutectoid cementite [8 marks] c. Cesium chloride (CsCl) is AX-type crystal structure. i. Draw it’s unit structure and state it’s coordination number ii. Demonstrate that the minimum cation-to-anion radius ration for that coordination number is 0.732. [8 marks] d. In your own words, describe what happens as a piece of glass is thermally tempered. [4 marks]
The Correct Answer and Explanation is:
Here are the solutions to the questions provided.
Part a
Sketches of the microstructures for the given iron-carbon alloys:
i. 0.25 wt%C at just below eutectoid temperature (Hypoeutectoid Steel)
This microstructure consists of two constituents: proeutectoid ferrite and pearlite. Since the carbon content is low, there is a large fraction of proeutectoid ferrite (the lighter, continuous phase) and a smaller fraction of pearlite (the dark, lamellar colonies).

ii. 0.76 wt%C at just above eutectoid temperature (Eutectoid Steel)
At the eutectoid composition (0.76 wt%C) and a temperature just above the eutectoid line, the alloy is entirely in the austenite phase field. The microstructure consists of 100% austenite grains.

iii. 1.3 wt%C at just below eutectoid temperature (Hypereutectoid Steel)
This microstructure consists of proeutectoid cementite and pearlite. The proeutectoid cementite (light, thin network) forms along the prior austenite grain boundaries. The remaining austenite transforms into pearlite (dark, lamellar colonies) which fills the grains.

Part b
Calculations for a 1.3 wt%C steel at just below eutectoid temperature. The relevant compositions are:
- Overall alloy composition, C₀ = 1.3 wt%C
- Composition of ferrite (α), Cα ≈ 0.022 wt%C
- Composition of cementite (Fe₃C), C_Fe₃C = 6.70 wt%C
- Eutectoid composition, C_eutectoid = 0.76 wt%C
i. The fractions of total ferrite and cementite phases
Using the lever rule on the α + Fe₃C phase field:
- Weight Fraction of total Ferrite (Wα):
Wα = (C_Fe₃C – C₀) / (C_Fe₃C – Cα) = (6.70 – 1.3) / (6.70 – 0.022) = 5.4 / 6.678 ≈ 0.809 (or 80.9%) - Weight Fraction of total Cementite (W_Fe₃C):
W_Fe₃C = (C₀ – Cα) / (C_Fe₃C – Cα) = (1.3 – 0.022) / (6.70 – 0.022) = 1.278 / 6.678 ≈ 0.191 (or 19.1%)
ii. The fractions of the proeutectoid and pearlite phases
Using the lever rule just above the eutectoid temperature on the γ + Fe₃C phase field:
- Weight Fraction of Proeutectoid Cementite (W_Fe₃C’):
This is the fraction of the cementite phase that forms before the eutectoid reaction.
W_Fe₃C’ = (C₀ – C_eutectoid) / (C_Fe₃C – C_eutectoid) = (1.3 – 0.76) / (6.70 – 0.76) = 0.54 / 5.94 ≈ 0.091 (or 9.1%) - Weight Fraction of Pearlite (Wp):
This is the fraction of austenite that transforms into pearlite at the eutectoid temperature.
Wp = (C_Fe₃C – C₀) / (C_Fe₃C – C_eutectoid) = (6.70 – 1.3) / (6.70 – 0.76) = 5.4 / 5.94 ≈ 0.909 (or 90.9%)
iii. The fraction of eutectoid cementite
Eutectoid cementite is the cementite contained within the pearlite microconstituent.
- First, find the fraction of cementite within pearlite:
W_Fe₃C(in pearlite) = (C_eutectoid – Cα) / (C_Fe₃C – Cα) = (0.76 – 0.022) / (6.70 – 0.022) = 0.738 / 6.678 ≈ 0.1105 - Then, multiply by the total fraction of pearlite in the alloy:
Fraction of eutectoid cementite = W_Fe₃C(in pearlite) × Wp = 0.1105 × 0.909 ≈ 0.100 (or 10.0%)
Part c
i. Draw its unit structure and state its coordination number
The Cesium Chloride (CsCl) unit cell has a simple cubic structure. It consists of anions (e.g., Cl⁻) at the eight corners of a cube and a cation (e.g., Cs⁺) at the body center.

The coordination number is 8. Each central Cs⁺ ion is in contact with the 8 corner Cl⁻ ions, and each Cl⁻ ion is shared by eight unit cells, making it equidistant from 8 body-centered Cs⁺ ions.
ii. Demonstrate that the minimum cation-to-anion radius ratio for that coordination number is 0.732
For a stable structure with a coordination number of 8, the central cation must touch the corner anions. The minimum radius ratio occurs when the corner anions also touch each other along the cube edge.
Let r_C be the cation radius and r_A be the anion radius. Let ‘a’ be the lattice parameter (cube edge length).
- Anion-Anion Contact: At the minimum ratio, the anions at the corners touch along the cube edge ‘a’.
a = r_A + r_A = 2r_A - Cation-Anion Contact: The cation at the body center touches the anions at the corners along the body diagonal of the cube. The length of the body diagonal is a√3. The distance from the center to a corner is half this length.
r_C + r_A = (a√3) / 2 - Demonstration: Substitute the expression for ‘a’ from step 1 into the equation from step 2.
r_C + r_A = (2r_A * √3) / 2
r_C + r_A = r_A * √3
r_C = r_A * √3 – r_A
r_C = r_A(√3 – 1) - Calculate the Ratio:
r_C / r_A = √3 – 1
Since √3 ≈ 1.732,
r_C / r_A ≈ 1.732 – 1 = 0.732
Part d
Thermal tempering is a process used to increase the strength and safety of glass. It involves a controlled heating and cooling cycle that induces permanent stress into the material.
First, the glass is heated uniformly to a temperature above its glass transition temperature, typically around 600°C. At this point, the glass is soft enough to relieve any existing internal stresses from its manufacturing.
Immediately after heating, the outer surfaces of the glass are rapidly cooled by jets of cold air. This process is called quenching. The surfaces cool down much faster than the interior, causing them to contract and solidify first, forming a rigid outer shell.
As the hot, molten-like interior then begins to cool down more slowly, it also attempts to contract. However, it is constrained by the already solid outer layers. This pulling action from the cooling interior places the rigid surfaces into a state of high compression, while the interior is forced into a balancing state of tension.
This final stress distribution is the key to the glass’s enhanced properties. Glass is inherently weak in tension but very strong in compression. The induced surface compression must be overcome by an applied tensile force before the surface can experience any net tension, which is required for cracks to propagate. This makes tempered glass several times stronger than ordinary annealed glass. A significant safety feature is its fracture pattern; when it breaks, the stored strain energy is released at once, causing the entire pane to shatter into many small, granular, and relatively harmless pieces instead of dangerous sharp shards.thumb_upthumb_down
