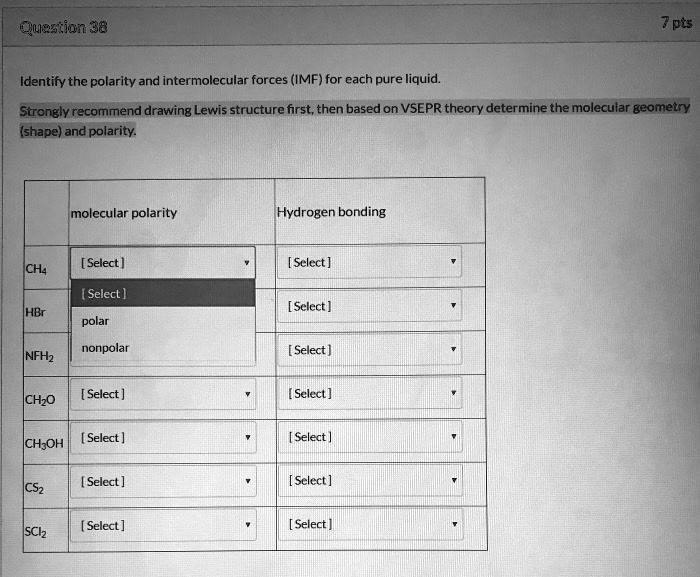
Question 38 Identify the polarity and intermolecular forces (IMF) for each pure liquid. Strongly recommend drawing Lewis structure first, then based on VSEPR theory determine the molecular geometry (shape) and polarity: Molecular Polarity: Hydrogen bonding: [Select] CH4 [Select] HBr [Select] polar nonpolar [Select] NF3 [Select] CH2O [Select] [Select] CH3OH [Select] [Select] CS2 [Select] [Select] SCl2
The Correct Answer and Explanation is:
Here are the correct selections for each molecule based on its polarity and ability to form hydrogen bonds.
CH₄ (Methane)
- Molecular Polarity: nonpolar
- Hydrogen bonding: no
HBr (Hydrogen Bromide)
- Molecular Polarity: polar
- Hydrogen bonding: no
NF₃ (Nitrogen Trifluoride)
- Molecular Polarity: polar
- Hydrogen bonding: no
CH₂O (Formaldehyde)
- Molecular Polarity: polar
- Hydrogen bonding: no
CH₃OH (Methanol)
- Molecular Polarity: polar
- Hydrogen bonding: yes
CS₂ (Carbon Disulfide)
- Molecular Polarity: nonpolar
- Hydrogen bonding: no
SCl₂ (Sulfur Dichloride)
- Molecular Polarity: polar
- Hydrogen bonding: no
Explanation
The polarity of a molecule is determined by both the polarity of its individual bonds and its overall molecular geometry. A molecule with polar bonds can be nonpolar if its shape is symmetrical, causing the bond dipoles to cancel out. Hydrogen bonding, a strong type of intermolecular force, occurs in pure substances where molecules contain a hydrogen atom covalently bonded to a highly electronegative atom—specifically nitrogen (N), oxygen (O), or fluorine (F).
- CH₄ (Methane): Methane has a symmetrical tetrahedral geometry. Although the C-H bonds have a very small electronegativity difference, their symmetrical arrangement causes the bond dipoles to cancel, making the molecule nonpolar. It lacks H bonded to N, O, or F, so it does not exhibit hydrogen bonding.
- HBr (Hydrogen Bromide): As a diatomic molecule with a significant electronegativity difference between hydrogen and bromine, the H-Br bond is polar. This makes the entire molecule polar. Hydrogen is bonded to bromine, not N, O, or F, so there is no hydrogen bonding.
- NF₃ (Nitrogen Trifluoride): The N-F bonds are polar. Due to a lone pair on the central nitrogen atom, the molecule has a trigonal pyramidal shape. This asymmetrical geometry prevents the bond dipoles from canceling, making NF₃ polar. It does not contain hydrogen, so it cannot hydrogen bond.
- CH₂O (Formaldehyde): The C=O double bond is very polar. The molecule’s trigonal planar geometry is asymmetrical because the C=O and C-H bonds are different. Thus, there is a net dipole moment, and the molecule is polar. Hydrogen is bonded to carbon, not oxygen, so pure formaldehyde does not form hydrogen bonds.
- CH₃OH (Methanol): The molecule contains polar C-O and O-H bonds and has an asymmetrical structure, making it polar. Crucially, it has an O-H group, which allows methanol molecules to act as both hydrogen bond donors and acceptors. Therefore, it does exhibit hydrogen bonding.
- CS₂ (Carbon Disulfide): The molecule has a linear, symmetrical structure (S=C=S). Any small polarity in the C-S bonds is canceled out, making the molecule nonpolar. It contains no hydrogen atoms, so it cannot hydrogen bond.
- SCl₂ (Sulfur Dichloride): The S-Cl bonds are polar. The central sulfur atom has two lone pairs, giving the molecule a bent geometry. This asymmetrical shape results in a net dipole moment, making SCl₂ polar. It has no hydrogen atoms and thus cannot hydrogen bond.
