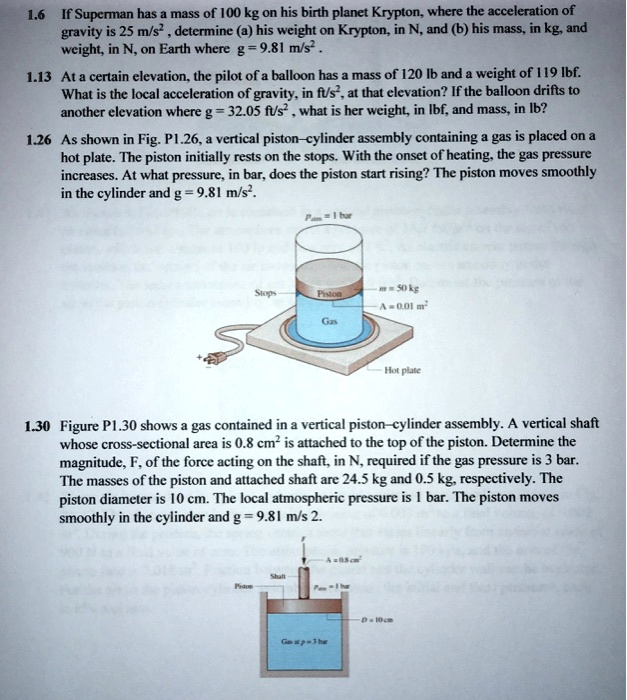
If Superman has a mass of 100 kg on his birth planet Krypton, where the acceleration of gravity is 25 m/s², determine (a) his weight on Krypton, in N, and (b) his mass, in kg and weight, in N, on Earth where g = 9.81 m/s². 1.13 At a certain elevation, the pilot of a balloon has a mass of 120 lb and weight of 119 lb. What is the local acceleration of gravity in ft/s² at that elevation? If the balloon drifts to another elevation where g = -32.05 ft/s², what is her weight in lb and mass in lb? 1.26 As shown in Fig: PL.26, a vertical piston-cylinder assembly containing gas is placed on a hot plate. The piston initially rests on the stops. With the onset of heating, the gas pressure increases. At what pressure, in bar, does the piston start rising? The piston moves smoothly in the cylinder and g = 9.81 m/s². 130 Figure PI.30 shows gas contained in a vertical piston-cylinder assembly. A vertical shaft whose cross-sectional area is 0.8 cm² is attached to the top of the piston. Determine the magnitude F of the force acting on the shaft, in N, required if the gas pressure is 3 bar. The masses of the piston and attached shaft are 24.5 kg and 0.5 kg, respectively. The piston diameter is 10 cm. The local atmospheric pressure is 1 bar. The piston moves smoothly in the cylinder and g = 9.81 m/s².
The Correct Answer and Explanation is:
Correct Answer for Problem 1.26: The gas pressure must be 1.4905 bar for the piston to start rising.
Explanation
This problem requires us to determine the internal gas pressure needed to overcome the downward forces acting on the piston. The piston will begin to rise at the exact moment the upward force exerted by the gas equals the total downward force. This is a static equilibrium problem.
The forces acting on the piston are:
- Downward Force from Piston’s Weight (W): This is the mass of the piston multiplied by the acceleration due to gravity.
W = m * g - Downward Force from Atmospheric Pressure (F_atm): The atmosphere above exerts a pressure on the top surface of the piston. This force is the atmospheric pressure multiplied by the piston’s area.
F_atm = P_atm * A - Upward Force from Gas Pressure (F_gas): The heated gas inside the cylinder exerts an upward pressure on the bottom surface of the piston. This force is the gas pressure multiplied by the piston’s area.
F_gas = P_gas * A
For the piston to lift, the upward force must balance the sum of the downward forces:
F_gas = W + F_atm
P_gas * A = (m * g) + (P_atm * A)
We can solve for the gas pressure (P_gas) by dividing the entire equation by the area (A):
P_gas = P_atm + (m * g) / A
Now, we substitute the given values, ensuring consistent units. We will convert all pressure values to Pascals (Pa) for the calculation, since 1 N/m² = 1 Pa.
- m = 50 kg
- g = 9.81 m/s²
- A = 0.01 m²
- P_atm = 1 bar = 100,000 Pa
First, calculate the pressure exerted by the piston’s weight:
Pressure from weight = (50 kg * 9.81 m/s²) / 0.01 m² = 490.5 N / 0.01 m² = 49,050 Pa
Next, add the atmospheric pressure to find the total required gas pressure in Pascals:
P_gas = 100,000 Pa + 49,050 Pa = 149,050 Pa
Finally, convert this pressure back to bar (1 bar = 100,000 Pa):
P_gas = 149,050 Pa / 100,000 Pa/bar = 1.4905 bar
