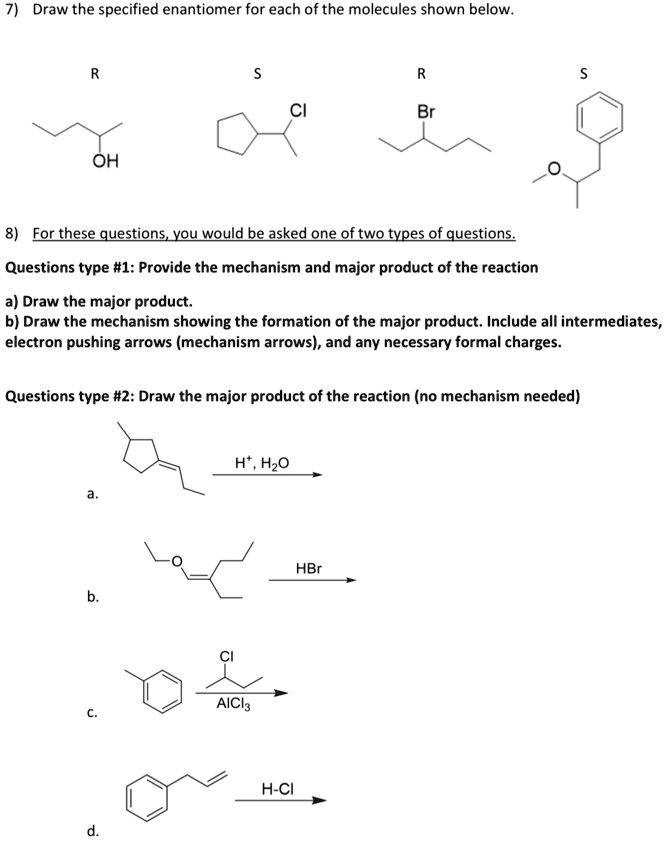
Draw the specified enantiomer for each of the molecules shown below.
The Correct Answer and Explanation is:
Here are the correct answers for the provided chemistry problems.
Answer to Question 7
The specified enantiomers are drawn below, with the chiral center’s stereochemistry indicated using wedge and dash notation.
Answer to Question 8
The major organic products for each reaction are drawn below.
Explanation
Question 7: Drawing Enantiomers
This question requires drawing the specific three-dimensional structure of a chiral molecule. To do this, we use the Cahn-Ingold-Prelog (CIP) rules to assign priorities (1-4, with 1 being the highest) to the four groups attached to the chiral center based on atomic number. For an R configuration, when the lowest priority group (4) is pointing away (dashed bond), the sequence from 1 → 2 → 3 is clockwise. For an S configuration, the sequence is counter-clockwise. For example, in (R)-butan-2-ol, the priorities are (1) -OH, (2) -CH₂CH₃, (3) -CH₃, and (4) -H. Placing the -H group on a dash and arranging the other groups in a clockwise 1-2-3 sequence gives the correct R enantiomer.
Question 8: Predicting Major Products
a. This reaction is an acid-catalyzed hydration of an alkene. According to Markovnikov’s rule, the initial protonation of the double bond occurs at the less substituted carbon to form the most stable carbocation intermediate. The double bond carbon within the ring is more substituted (tertiary center) than the exocyclic carbon (secondary center). Therefore, the proton adds to the exocyclic carbon, creating a stable tertiary carbocation on the ring. The water molecule then acts as a nucleophile, attacking this carbocation to form the tertiary alcohol, 2-propyl-1-methylcyclopentan-2-ol, as the major product.
b. This reaction involves the acid-catalyzed cleavage of a vinyl ether (enol ether) with HBr. The double bond is first protonated to form a resonance-stabilized oxocarbenium ion. The bromide ion (Br⁻) then acts as a nucleophile. Instead of adding to the double bond carbon, it attacks the ethyl group attached to the oxygen in an SN2-like process. This cleaves the ether, yielding two products: an alkyl halide (bromoethane) and an enol. The enol intermediate is unstable and rapidly tautomerizes to its more stable keto form, which is 3-methylpentan-2-one, the major organic product.
c. This is a Friedel-Crafts alkylation reaction. The Lewis acid, AlCl₃, reacts with tert-butyl chloride to generate a stable tert-butyl carbocation. This carbocation is a strong electrophile that attacks the electron-rich toluene ring. The methyl group on toluene is an activating, ortho, para-director. However, due to the significant steric bulk of the tert-butyl group, attack at the para position is heavily favored over the sterically hindered ortho positions. Thus, the major product is 1-tert-butyl-4-methylbenzene.
d. This is an electrophilic addition of HCl to an alkene. Protonation of the double bond occurs at the terminal carbon (C1) to form a secondary carbocation at C2, following Markovnikov’s rule. However, this secondary carbocation can rearrange via a 1,2-hydride shift from the adjacent benzylic carbon (C3). This shift results in a more stable secondary benzylic carbocation, which is stabilized by resonance with the phenyl ring. The chloride ion then attacks this rearranged, more stable carbocation, leading to the formation of 1-chloro-1-phenylpropane as the major product
