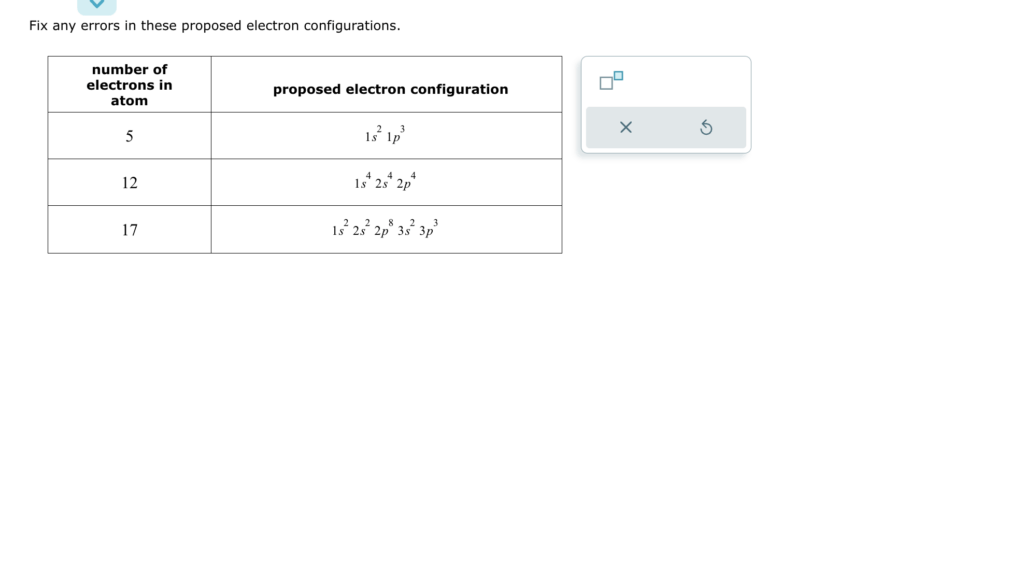
Fix any errors in these proposed electron configurations.
The Correct Answer and Explanation is:
Here are the corrected electron configurations for the atoms with the given number of electrons:
- For 5 electrons (Boron, B):
The proposed configuration is: 1s² 1p³. The correct configuration is: 1s² 2p³.
Explanation: Boron has 5 electrons. The first two electrons fill the 1s orbital, and the remaining three electrons go into the 2p orbital, since the 1p orbital does not exist. - For 12 electrons (Magnesium, Mg):
The proposed configuration is: 1s⁴ 2s⁴ 2p⁴. The correct configuration is: 1s² 2s² 2p⁶.
Explanation: Magnesium has 12 electrons. The 1s orbital can hold a maximum of 2 electrons, and the 2s and 2p orbitals can each hold 2 and 6 electrons respectively. The proposed configuration had incorrect superscripts for each orbital. - For 17 electrons (Chlorine, Cl):
The proposed configuration is: 1s² 2s² 2p⁸ 3s² 3p³. The correct configuration is: 1s² 2s² 2p⁶ 3s² 3p⁵.
Explanation: Chlorine has 17 electrons. The 1s, 2s, and 2p orbitals are filled, with 2p holding 6 electrons. The remaining 7 electrons fill the 3s (2 electrons) and 3p (5 electrons) orbitals. The proposed configuration had an incorrect number of electrons in the 2p orbital.
Summary of Errors:
- The 1p orbital is nonexistent.
- The superscripts indicating the number of electrons in each orbital were incorrect for magnesium and chlorine.
- Electrons should be placed in orbitals in the proper order, filling 1s before 2s, 2s before 2p, and so on.
This illustrates the importance of following the order of orbital filling and ensuring the correct number of electrons for each orbital.
