The Correct Answer and Explanation is:1
Let’s go over the proposed electron configurations for the atoms with 28, 20, and 18 electrons.
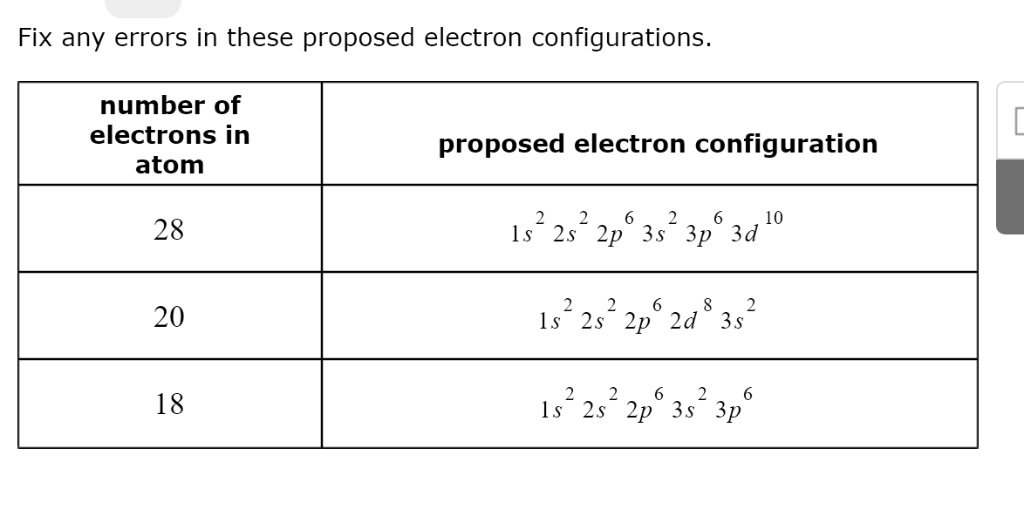
- 28 Electrons:
The electron configuration provided is:1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰.
However, this configuration is incorrect. The total electron count is only 26, as the 3d subshell should contain 10 electrons (correct), but the 4s subshell is missing.
The correct configuration should be:1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s².
This configuration represents the atom of Nickel (Ni), with 28 electrons in total. - 20 Electrons:
The configuration provided is:1s² 2s² 2p⁶ 2d⁸ 3s² 3p².
This is incorrect because the 2d subshell does not exist. The valid subshells are 2s, 2p, 3s, 3p, etc., and there is no 2d orbital. The correct configuration should fill the available subshells properly, and the 3d subshell comes after the 4s subshell.
The correct configuration for an atom with 20 electrons (such as Calcium (Ca)) is:1s² 2s² 2p⁶ 3s² 3p⁶ 4s². - 18 Electrons:
The configuration provided is:1s² 2s² 2p⁶ 3s² 3p⁶.
This configuration is correct for an atom with 18 electrons, which corresponds to the element Argon (Ar). Argon is a noble gas, and its electron configuration fills all lower subshells to their maximum capacity without further electron placement in the higher energy levels.
Summary of Corrected Configurations:
- 28 electrons:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² - 20 electrons:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² - 18 electrons:
1s² 2s² 2p⁶ 3s² 3p⁶
These corrections align with the correct subshells and the filling order of orbitals.
