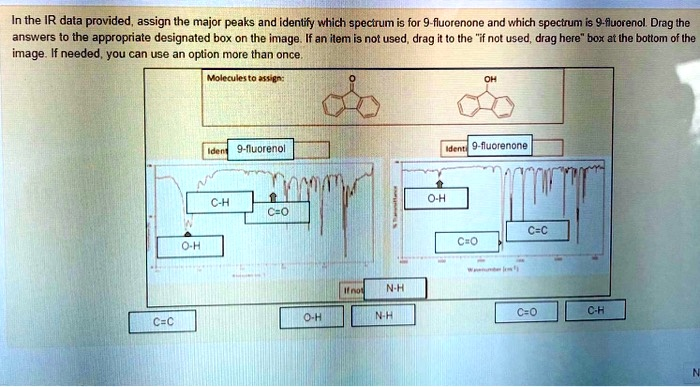
In the IR data provided, assign the major peaks and identify which spectrum is for 9-fluorenone and which spectrum is 9-fluorenol. Drag the answers to the appropriate designated box on the image. If an item is not used, drag it to the “if not used, drag here” box at the bottom of the image. If needed, you can use an option more than once. O-H Molecules to assign: Ident 9-fluorenol C-H O-H C=O If not N-H C=C O-H N-H OH Identi 9-fluorenone C=C C=O C=O C-H N
The Correct Answer and Explanation is:
Based on the principles of IR spectroscopy, here is the correct assignment for the given spectra and molecules.
Left Spectrum:
- Molecule Identification: 9-fluorenone
- Peak Assignments:
- C-H: The box points to the sharp peaks just above 3000 cm⁻¹, which correspond to the aromatic C-H stretches.
- C=O: The box points to the very strong, sharp absorption at approximately 1715 cm⁻¹. This is the characteristic peak for a ketone’s carbonyl (C=O) group. The conjugation with the aromatic rings lowers the frequency slightly from a simple aliphatic ketone.
- The box pointing to the baseline around 3300 cm⁻¹ should not have a label, as there is no O-H peak present.
Right Spectrum:
- Molecule Identification: 9-fluorenol
- Peak Assignments:
- O-H: The box points to the very broad, strong peak centered around 3300 cm⁻¹. This is the classic signature of an alcohol’s O-H stretching vibration.
- C=C: The box points to the absorptions in the 1450-1600 cm⁻¹ region, which are characteristic of carbon-carbon double bond stretching within the aromatic rings.
- The box pointing to the baseline around 1700 cm⁻¹ should not have a label, as the absence of a peak here confirms the C=O group is not present.
Unused Labels:
- N-H: Neither 9-fluorenone nor 9-fluorenol contains a nitrogen-hydrogen bond, so this label is not used.
Explanation
The key to distinguishing between the IR spectra of 9-fluorenone and 9-fluorenol is to look for the characteristic peaks of their different functional groups: a ketone (C=O) for 9-fluorenone and an alcohol (O-H) for 9-fluorenol.
- Identifying 9-fluorenol: The spectrum on the right displays a very prominent, broad, and strong absorption band in the region of 3200-3600 cm⁻¹. This is the definitive signal for the O-H stretching vibration of an alcohol. The broadness is due to hydrogen bonding between the alcohol molecules. This spectrum also lacks a strong peak around 1700 cm⁻¹, confirming the absence of a carbonyl group. Therefore, the right spectrum belongs to 9-fluorenol. The peaks around 1600 cm⁻¹ are correctly assigned to the C=C bonds of the aromatic rings.
- Identifying 9-fluorenone: The spectrum on the left lacks the broad O-H peak seen in the other spectrum. Instead, it shows a very strong, sharp absorption peak at approximately 1715 cm⁻¹. This is the characteristic position for a C=O (carbonyl) stretch in a conjugated ketone. The peaks just above 3000 cm⁻¹ are due to the C-H stretching of the aromatic rings. The absence of the O-H peak and the presence of the strong C=O peak definitively identify the left spectrum as belonging to 9-fluorenone.
- Unused Label: Both molecules are hydrocarbons with an oxygen atom, but neither contains nitrogen. Thus, the N-H label is not applicable to either spectrum and should be placed in the “if not used” category.
