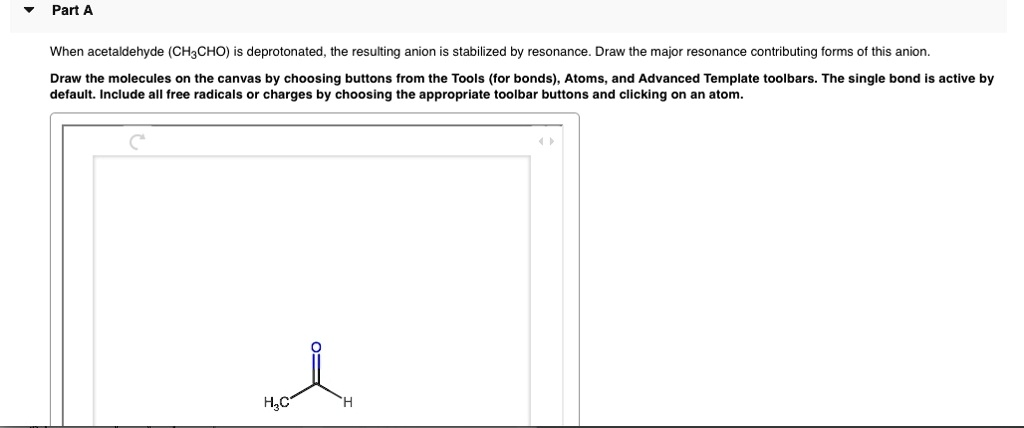
Part A When acetaldehyde (CH3CHO) deprotonated, the resulting anion stabilized by resonance_ Draw the major resonance contributing forms of this anion: Draw the molecules on the canvas by choosing buttons from the Tools (for bonds) , Atoms and Advanced Template toolbars. The single bond is active by default: Include all Iree radicals or charges by choosing the appropriate toolbar buttons and clicking on an atom_
The Correct Answer and Explanation is:
To draw the resonance structures of the acetaldehyde anion (CH3CHO), after it has been deprotonated, we need to account for the negative charge on the oxygen atom, which typically occurs when the molecule loses a proton (H+) from the –OH group of the aldehyde functional group. Here’s a step-by-step explanation:
- Initial deprotonation: The proton (H) is removed from the hydroxyl group (-OH) in acetaldehyde. This creates a negatively charged oxygen atom (O−). The remaining species is CH3C(O−)H.
- Resonance forms: The oxygen atom with the negative charge can delocalize its charge through resonance. The lone pair of electrons on oxygen can be transferred to form a double bond between the oxygen and the carbonyl carbon. This results in a negative charge on the carbonyl carbon.
- Resonance structures: The first resonance structure has the negative charge on the oxygen atom, and in the second resonance structure, the negative charge is delocalized to the carbonyl carbon. The oxygen can also shift its electron pair back to the oxygen, resulting in a double bond and restoring the negative charge on the oxygen.
- Key structures:
- One form shows a single bond between the oxygen and carbon, with a negative charge on the oxygen.
- The second form shows a delocalized negative charge, with the carbonyl carbon having a negative charge while oxygen maintains the double bond.
These resonance structures stabilize the anion, as the negative charge is shared between oxygen and carbon in the form of resonance.
In summary, the major resonance structures of the deprotonated acetaldehyde anion involve the delocalization of the negative charge between the oxygen atom and the carbonyl carbon through resonance. This stabilizes the anion, as both atoms (oxygen and carbon) can partially share the negative charge.
